حراجناموجود

HCV Real-TM Quant DX Real-TM,sacace,96 t
59,727,614 تومان 49,773,012 تومان
HIV Real-TM Quant DX Real-TM,sacace ,96 t
63,347,469 تومان 52,789,558 تومان
ناموجود
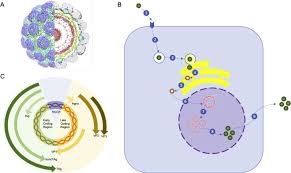
HIV Real-TM Quant Dx Handbook Real Time PCR Kit for the Quantitative detection of Human Immunodeficiency Virus (HIV-1) in human plasma REF V0-96/3FRT 96 Sacace Biotechnologies Srl via Scalabrini, 44 – 22100 – Como – Italy Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 2 KEY TO SYMBOLS USED List Number Contains sufficient for tests Lot Number Version RUOFor Research Use Only CONTROL INT Internal control Temperature limitations CONTROL + High Positive Control Manufacturer CONTROL -N Negative Control Consult instructions for use CAL 1 Standard 1 Expiration Date CAL 2 Standard 2 Caution! Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 3 TABLE OF CONTENTS NAME ……………………………………………………………………………………………………………………………. 4 INTRODUCTION ………………………………………………………………………………………………………….. 4 INTENDED USE …………………………………………………………………………………………………………… 5 PRINCIPLE OF ASSAY ………………………………………………………………………………………………… 5 MATERIALS PROVIDED ……………………………………………………………………………………………… 6 MATERIALS REQUIRED BUT NOT PROVIDED ………………………………………………………….. 6 WARNINGS AND PRECAUTIONS ……………………………………………………………………………….. 7 STABILITY ……………………………………………………………………………………………………………………. 8 QUALITY CONTROL ……………………………………………………………………………………………………. 8 SAMPLE COLLECTION, STORAGE AND TRANSPORT …………………………………………….. 8 INTERFERING SUBSTANCES …………………………………………………………………………………….. 9 REAGENTS PREPARATION ……………………………………………………………………………………….. 9 RNA ISOLATION ………………………………………………………………………………………………………….. 9 INTERNAL CONTROL ………………………………………………………………………………………………… 10 ASSAY CALIBRATION ……………………………………………………………………………………………….. 10 ASSAY PROTOCOL ………………………………………………………………………………………………….. 10 RESULTS INTERPRETATION ……………………………………………………………………………………. 12 PERFORMANCE CHARACTERISTICS ……………………………………………………………………… 13 ANALYTICAL SENSITIVITY ……………………………………………………………………………………. 13 LINEAR RANGE ……………………………………………………………………………………………………… 13 CROSS-REACTIVITY ……………………………………………………………………………………………… 14 ROBUSTNESS ………………………………………………………………………………………………………… 14 CROSS CONTAMINATION …………………………………………………………………………………….. 14 PROTOCOL FOR ROTORGENE™ 6000/Q ……………………………………………………………….. 15 Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 4 NAME HIV Real-TM Quant Dx INTRODUCTION HIV is a lentivirus (a member of the retrovirus family) differentiated on a structural and antigenic properties into two types: HIV-1 and HIV-2. HIV-2 occurs considerably less often than HIV-1. Transmission ways of the virus are very important for the virus spread. HIV is transmitted by three ways: at heterosexual and homosexual intercourse, parenteral with blood and blood products and vertically, from the infected mother to the child by intrauterine way, during the child delivering or soon after the childbirth at breast feeding. HIV is transmitted as single-stranded, positive-sense, enveloped RNA viruses. Upon entry into the target cell, the viral RNA genome is converted (reverse transcribed) into double-stranded DNA by a virally encoded reverse transcriptase that is transported along with the viral genome in the virus particle. The resulting viral DNA is then imported into the cell nucleus and integrated into the cellular DNA by a virally encoded integrase and host co-factors. Once integrated, the virus may become latent, allowing the virus and its host cell to avoid detection by the immune system. Alternatively, the virus may be transcribed, producing new RNA genomes and viral proteins that are packaged and released from the cell as new virus particles that begin the replication cycle anew. One of the most effective current methods of direct HIV detection is specific amplification of nucleic acids in vitro by polymerase chain reaction (PCR). This method shows many advantages: detection of virus DNA/RNA allows reducing the length of the “serological window”; PCR is an indispensable approach for HIV-diagnostics in children born from HIV-infected mothers; determination of HIV RNA in the blood plasma (viral load) is an obligatory procedure to monitoring of the therapy effectiveness. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 5 INTENDED USE HIV Real-TM Quant Dx Kit is a Real-Time Amplification test for the Quantitative detection of Human Immunodeficiency Virus 1 (HIV-1) RNA in human plasma and the simultaneous detection of a HIVspecific Internal Control (IC), by dual color detection. Quantitative HIV RNA testing provides prognostic information regarding likelihood of treatment response to antiretroviral therapy. Quantitative measurement of HIV levels in peripheral blood has been shown to be an essential parameter in prognosis and management of HIV infected individuals. Decisions regarding initiation or changes in antiretroviral therapy are guided by monitoring plasma HIV RNA levels (viral load), CD4+ T cell count, and the patient’s clinical condition. The aim of antiretroviral therapy is to reduce the HIV RNA concentration in plasma to levels not detectable through available viral load assays. HIV Real-TM Quant Dx Real Time assay is not for screening blood, plasma, serum or tissue donors for HIV, or to be used as a diagnostic test to confirm the presence of HIV infection. PRINCIPLE OF ASSAY HIV Real-TM Quant Dx Kit is a Real-Time test for the Quantitative detection of Human Immunodeficiency Virus 1 (HIV-1) RNA in human plasma. HIV RNA is extracted from plasma, amplified using real time amplification and detected using fluorescent reporter dye probes specific for HIV or HIV IC. During each round of thermal cycling, amplification products dissociate to single strands at high temperature allowing primer annealing and extension as the temperature is lowered. Exponential amplification of the product is achieved through repeated cycling between high and low temperatures, resulting in a billion-fold or greater amplification of target sequences. Amplification of both targets (HIV and IC) takes place simultaneously in the same reaction. Monitoring the fluorescence intensities during Real Time allows the detection and quantification of the accumulating product without having to re-open the reaction tube after the real time amplification. Internal Control (IC) serves as an extraction and an amplification control for each individually processed specimen and to identify possible inhibition. IC is detected in a channel other than the HIV RNA. HIV-IC is a lyophilized Internal Control and represents recombinant RNA-containing-structure which carried through all steps of analysis from nucleic acid extraction to PCR amplification-detection. The presence of HIV Rec IC allows not only to monitor the extraction procedure and to check possible PCR inhibition but also to verify possible losses of the RNA during extraction procedure thus enabling to calculate precisely the HIV viral load. The assay is standardized against the 3rd HIV-1 WHO International Standard for Human Immunodeficiency Virus for Nucleic Acid Amplification Techniques (NIBSC code: 10/152) and results are reported in International Units/mL (IU/mL). The target sequence for the HIV Real-TM Quant Dx assay is the pol gene of the HIV genome. This region is specific for HIV and is highly conserved. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 6 MATERIALS PROVIDED Sacace HIV Real-TM Quant Dx Amplification Reagent Kit RT-PCR REAGENT PACK 96 vials (0,2 ml) with lyophilized amplification reagents, 0,1g Sacace HIV Real-TM Quant Dx Control Kit1, 2, 3 CONTROL INT 4 vials with lyophilized reagent HIV-IC-L, 0,2g CONTROL + 4 vials with lyophilized reagent HIV-Pos C+ – Sacace HIV Real-TM Quant Dx Positive Control, 0,2g CONTROL – 4 vials, 12,0 ml per vial with Negative Control RB BUFFER 4 vials, 4,0 ml per vial with Reconstitution Buffer (RB Buffer) Sacace HIV Real-TM Quant Dx Calibrator Kit1,2 CAL 1 2 vials with lyophilized reagent HIV Quantitative Standard 1, 0,2g CAL 2 2 vials with lyophilized reagent HIV Quantitative Standard 2, 0,2g 1Standards’ and controls’ concentrations are specific for every lot. 2must be used during the sample preparation procedure (see RNA isolation) 3The Control+ must be prepared exactly in the same way as the CAL1 and CAL2 reagents as indicated in both the REAGENTS PREPARATION and ASSAY CALIBRATION sections below. It must be extracted before Real Time PCR analysis. MATERIALS REQUIRED BUT NOT PROVIDED RNA isolation kit (see RNA isolation) Desktop microcentrifuge for “eppendorf” type tubes Vortex mixer Disposable gloves, powderless Biohazard waste container Refrigerator, Freezer Real Time Thermal cycler Biological safety cabinet approved for working with infectious materials Pipettes (adjustable) Sterile pipette tips with filters Tube racks Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 7 WARNINGS AND PRECAUTIONS RUO For Professional Use Only 1. Read the instructions in this package insert carefully before processing samples. Strict compliance with the user manual is required for optimal PCR results. 2. Wear disposable gloves, laboratory coats and eye protection when handling specimens and reagents. Thoroughly wash hands afterward. 3. Use routine laboratory precautions. Do not eat, drink, smoke, apply cosmetics, or handle contact lenses in laboratory work areas. Do not pipette by mouth. 4. Do not use a kit after its expiration date. 5. Do not mix reagents from different kits. 6. To reduce the risk of nucleic acid contamination due to aerosols formed during pipetting, pipettes with aerosol barrier tips must be used. Change aerosol barrier pipette tips between all liquid transfers. 7. During preparation of samples, compliance with good laboratory practices is essential to minimize the risk of cross-contamination between samples as well as the inadvertent introduction of nucleases into samples during and after the extraction procedure. Proper aseptic technique should always be used when working with RNA or DNA. 8. Use only powder-free gloves. 9. Dispose all specimens and unused reagents in accordance with local regulations. 10. Heparin has been shown to inhibit reaction. The use of heparinized specimens is not recommended. 11. Specimens may be infectious. Use Universal Precautions when performing the assay. 12. Specimens and controls should be prepared in a laminar flow hood. 13. Handle all materials containing specimens or controls according to Good Laboratory Practices in order to prevent cross-contamination of specimens or controls. 14. Clean and disinfect all spills of specimens or reagents using a disinfectant such as 0,5% sodium hypochlorite, or other suitable disinfectant. Follow by wiping down the surface with 70% ethanol. 15. Avoid contact of specimens and reagents with the skin, eyes and mucous membranes. If these solutions come into contact, rinse immediately with water and seek medical advice immediately. 16. This product does not contain potentially infectious components. Material Safety Data Sheets (MSDS) are available on request. 17. Use of this product should be limited to personnel trained in the techniques of amplification. 18. Workflow in the laboratory must proceed in a uni-directional manner, beginning in the Extraction Area and moving to the Amplification Area. Do not return samples, equipment and reagents in the area where you performed previous step. Personnel should be using proper anti-contamination safeguards when moving between areas. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 8 STORAGE AND SHIPPING INSTRUCTIONS All components of the HIV Real-TM Quant Dx PCR kit must be stored at +2-8ºC when not in use. All components of the HIV Real-TM Quant Dx PCR kit are stable until the expiration date on the label. The shelf life of reagents before and after the first use is the same, unless otherwise stated. Ship HIV Real-TM Quant Dx PCR kit at +2-8ºC. STABILITY HIV Real-TM Quant Dx Test is stable up to the expiration date indicated on the kit label. The product will maintain performance through the control date printed on the label. Exposure to light, heat or humidity may affect the shelf life of some of the kit components and should be avoided. Components stored under conditions other than those stated on the labels may not perform properly and may adversely affect the assay results. When a positive or negative control value is out of the expected range, it may indicate deterioration of the reagents. Associated test results are invalid and samples must be retested. Assay recalibration may be necessary. QUALITY CONTROL In accordance with Sacace’s ISO 13485-Certified Quality Management System, each lot is tested against predetermined specifications to ensure consistent product quality. SAMPLE COLLECTION, STORAGE AND TRANSPORT Note: Handle all specimens as if they are potentially infectious agents. Current studies refer to EDTA or Citrate Phosphate Dextrose (CDP) plasma as the most suitable sample materials for HIV detection. Therefore, we recommend the use of these materials with the HIV Real-TM Quant Dx. The internal validation of the HIV Real-TM Quant Dx assay has been performed using human CDP plasma samples. Sample materials other than plasma are not validated. HIV Real-TM Quant Dx assay is only for use with human plasma specimens* 1. EDTA or CDP tubes may be used with the HIV Real-TM Quant Dx. Follow sample tube manufacturer’s instructions. 2. Whole blood collected in EDTA or CDP should be separated into plasma and cellular components by centrifugation at 800-1600 x g for 20 min within six hours. The isolated plasma has to be transferred into a sterile polypropylene tube. Plasma may be stored at 2-8°C for an additional 3 days. Alternatively, plasma may be stored at -18°C for up to one month or 1 year when stored at -70°C. 3. Do not freeze whole blood. 4. Specimens anti-coagulated with heparin are unsuitable for this test. 5. Thaw frozen specimens at room temperature before using. 6. Whole blood must be transported at 2-25°C and processed within 6 hours of collection. Plasma may be transported at 2-8°C or frozen. 7. Transportation of clinical specimens must comply with country, federal, state and local regulations for the transport of etiologic agents. * Serum can be used also as starting material on some occasions. In this case the analytical sensitivity of the kit HIV Real-TM Quant Dx is the same, but the clinical sensitivity may be significantly decreased because of the precipitation of viral particles during the clot retraction phase of serum preparation. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 9 INTERFERING SUBSTANCES Elevated levels of bilirubin (≥15 mg/dl) and lipids (≥800 mg/dl) do not influence the system. Heparin (≥10 IU/ml) affects the PCR. Samples that have been collected in tubes containing heparin as an anticoagulant should not to be used. Also, samples of heparinized patients must not be used. REAGENTS PREPARATION Before starting any HIV Real-TM Quant Dx protocol prepare the following reagents: 1. Choose the requested quantity of lyophilized controls and calibrators and centrifuge briefly. 2. Add Reconstitution Buffer (RB buffer) as for table below: Lyophilized reagent RB Buffer, µl CAL 1 1000 CAL 2 1000 CONTROL + 1000 CONTROL INT 1000 3. When opening the vials of lyophilized reagent, discard the cap and the stopper, add the indicated volume of RB Buffer and close the vial with the new cap supplied with the kit. 4. Wait for 15 minutes at room temperature, gently agitate periodically without inverting the vial. 5. Dissolved reagents CAL1, CAL2, CONTROL + must be stored at 2-8 °C and always protected from light up to 30 days, or aliquoted at -20°C for longer periods (50 µl aliquots, for up to 4 months); dissolved reagent CONTROL INT must be stored at 2-8 °C for up to 30 days. RNA ISOLATION Any commercial RNA/DNA isolation kit, if IVD-CE validated for the specimen types indicated herein at the “SAMPLE COLLECTION, STORAGE AND TRANSPORT” paragraph, could be used. Sacace Biotechnologies recommends to use the following kits: Magno-Virus (Sacace, REF K-2-16): sample volume 1000 µl, elution 50 µl SaMag Viral Nucleic Acids Extraction kit (Sacace, REF SM003): automated nucleic acid purification system: Kit sample volume 400 µl, elution 50 µl Please carry out the RNA extraction according to the manufacturer’s instructions. Add 30 μl of CONTROL INT during the RNA isolation procedure directly to the sample/lysis mixture in all samples, controls, calibrators (see Internal Control). Positive Control as well as Neg. Control (CONTROL -) must be always used with patient specimens during RNA isolation procedure. These controls must be treated in the same way as patient specimens following the Handbook of used RNA purification kit. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 10 INTERNAL CONTROL HIV-IC-L represents recombinant RNA-containing-structure which carried through all steps of analysis from nucleic acid extraction to PCR amplification-detection. The presence of HIV-IC-L allows not only to monitor the extraction procedure and to check possible PCR inhibition but also to verify possible losses of the RNA during extraction procedure thus enabling to calculate precisely the HIV viral load. An IC threshold cycle [Ct] assay validity parameter is established during a calibration run. A defined, consistent quantity of IC (30 μl) is introduced into each specimen, calibrator, and control at the beginning of sample preparation and measured on the real time PCR instrument to demonstrate proper specimen processing and assay validity. The median amplification cycle at which the IC target sequence fluorescent signal is detected during the calibration procedure (two calibrators are run in replicates of three) establishes an IC Ct validity range to be met by all subsequent processed specimens. The established range of samples IC Ct = Medium IC Ct of 6 calibrators ± 2 cycles Specimens whose IC Ct value exceeds the established range must be retested starting from sample preparation. ASSAY CALIBRATION A calibration curve is required to quantitate the HIV RNA concentration of specimens and controls. Two assay calibrators are run in replicates of three to generate a calibration curve (HIV concentration versus the threshold cycle [Ct] at which a reactive level of fluorescent signal is detected). The calibration curve slope and intercept are calculated and stored on the instrument. The concentration of HIV RNA in a sample is calculated from the stored calibration curve. Before the first use of a new lot of HIV Real-TM Quant Dx, 6 calibrators run must be performed beginning from RNA extraction procedure to generate a calibration curve (two calibrators are run in replicates of three): Prepare 3 new extraction tubes for CAL1, 3 new extraction tubes for CAL2 for each calibration run. Add 30 µl of CONTROL INT to each tube; Add 30 µl of CAL1 or CAL2 prepared in “REAGENTS PREPARATION” step to the appropriate tubes and then add the necessary volume of Negative Control (Control -) in order to reach the starting volume indicated in the manual of RNA purification kit. For example, for SaMag Viral 400 µl extraction, add 30 µl of CAL1 (or CAL2) prepared in “REAGENTS PREPARATION” step and then add 370 µl of Negative Control (SaMag Viral Nucleic Acids extraction kit from Sacace, REF SM003) Follow the sample preparation procedure described in the Handbook of used RNA purification kit. The prepared quantitative standards CAL1 and CAL2 must be treated in the same way as patient specimens in the extraction process. NOTE 1: the elution volume must be 50 µl regardless of the RNA purification kit used. NOTE 2: if the starting volume is different from default SaMag Viral kit (400 µl), the calculated results for samples must be adjusted using the following formula (‘Extraction Volume’ is the starting extraction volume): Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 11 ASSAY PROTOCOL Note: Reaction Mix volume = 50 µl No attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution REAL TIME PCR CALIBRATION PROCEDURE: 1. Prepare 6 PCR reaction tubes with lyophilized reagents to perform PCR of extracted calibrators. 2. Add 50 µl of eluted samples obtained from RNA purification of 6 calibrators. Follow the procedure for amplification and detection as described in this manual. Once an HIV Real-TM Quant Dx calibration run is accepted and stored, it may be used until the lot in use expires. During this time, all subsequent samples may be tested without further calibration by importing the experiment with Calibration Curve in the experiment with clinical samples using the same HIV RealTM Quant Dx lot number. REAL TIME PCR SAMPLE PROCEDURE: 1. Prepare requested quantity of reaction tubes with lyophilized reagents to perform PCR of extracted samples and controls. 2. Add 50 µl of eluted samples obtained from RNA purification step. Close the tubes and transfer them into the Real Time PCR instrument. Create a temperature profile on your Real-time instrument as follows: Rotor type instruments1 Plate type or modular instruments2 Stage Тemp, °С Time Fluorescence detection Cycle repeats Тemp, °С Time Fluorescence detection Cycle repeats Hold 45 30 min – 1 45 30 min – 1 Hold 94 1 min – 1 94 1 min – 1 Cycling 94 10 sec – 50 94 10 sec – 50 60 40 sec FAM(Green), ROX(Orange) 60 25 sec FAM(Green), ROX(Orange) 1 For example Rotor-Gene™ 3000/6000/Q (Corbett Research, Qiagen) 2 For example, SaCycler-96™ (Sacace) Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 12 QUALITY CONTROL PROCEDURE A defined quantity of Internal Control (IC) is introduced into each sample at the beginning of sample preparation procedure in order to control the extraction process of each individual sample and to identify possible reaction inhibition. One replicate each of the Negative Control (CONTROL -) and the HIV positive Control (CONTROL +) must be included in each test run. If the controls are out of their expected range (see Data Sheet), all of the specimens and controls from that run must be processed beginning from the sample preparation step. RESULTS INTERPRETATION The Internal Control (IC) is detected on FAM/Green channel and HIV RNA on ROX/Orange channel. Each DNA amplification is associated with generation of a fluorescence signal measurable in FAM/Green channel (for IC) or in ROX/Orange channel (for HIV RNA) resulting in a sigmoid growth curve (log scale). The data analysis is performed according to manufacturer’s instructions (Operation manual Rotor-Gene™ 6000/Q, Qiagen and Instrument Manual SaCycler-96™, Sacace) using the respective software and considering the recommendations of the handbook. Check the obtained results to ensure that the run is valid and to interpret results. HIV RNA is determined basing on CT values and as standard curve resulting from analysis of quantitation standards. HIV RNA concentration is expressed in IU/ml. If the result is more than 50,000,000 IU/ml, then it will be displayed as the result more than 50,000,000 IU HIV/ml. If the result is more than the linear measurement range, the sample can be analyzed after 10x dilution and the obtained result should be multiplied by 10. If the result is less than 48 IU/ml in case of extraction from 1 ml, then it will be displayed as the result less than 48 IU HIV/ml. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 13 PERFORMANCE CHARACTERISTICS The HIV Real-TM Quant Dx Kit was evaluated according to the common technical specifications (CTS) for in vitro diagnostic medical devices (2009/108/EC) and European Pharmacopoeia 7.0, p. 2.6.21. Nucleic acid amplification techniques (07/2010:20621). ANALYTICAL SENSITIVITY The analytical sensitivity or Limit of Detection (LOD) is defined as the smallest amount of HIV RNA that can be precisely detected with a probability of 95% or greater. The LOD in consideration of RNA purification from the sample is determined using HIV-positive clinical specimens in combination with a particular extraction method. HIV RNA was extracted with Magno-Virus purification kit (Sacace, REF K-2- 16) according to the manufacturer’s instructions. The LOD of the HIV Real-TM Quant Dx assay is 48 IU/mL with the 1.0 mL sample preparation procedure. The LOD was determined by testing dilutions of the 3rd HIV-1 WHO International Standard for Human Immunodeficiency Virus for Nucleic Acid Amplification Techniques (NIBSC code: 10/152) prepared in HIV negative human plasma. The results were determined by Probit analysis. LINEAR RANGE Linear range or Linearity of an assay is its ability to obtain test results that are directly proportional to the concentration of the nucleic acid. In other words, it is the interval between the upper and the lower concentration where test results are directly proportional to the concentrations. The upper limit of quantitation for the HIV Real-TM Quant Dx assay is 50 million IU/mL and the lower limit of quantitation is equal to the Limit of Detection (48 IU/mL with the 1.0 mL sample preparation procedure). The linear range of the HIV Real-TM Quant Dx kit was determined by analyzing a dilution series (from 8,00 log IU/ml to 1,00 log IU/ml) of an HIV synthetic quantitative standard calibrated against the 3rd HIV-1 WHO International Standard for Human Immunodeficiency Virus. SPECIFICITY The analytical specificity of the primers and the probes was validated with 100 negative plasma samples obtained from the European Directorate for the Quality of Medicine & HealthCare (EDQM). They did not generate any signal with the specific HIV primers and probes. The specificity of the kit HIV Real-TM Quant Dx was 100%. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 14 CROSS-REACTIVITY The potential cross-reactivity of the kit HIV Real-TM Quant Dx was tested against the group control listed in the following table. It was not observed any cross-reactivity with these pathogens. Table 1: Testing the specificity of the kit with other pathogens: Control group Results HIV Results IC Cytomegalovirus (CMV) – + Epstein Barr virus (EBV) – + Hepatitis B virus (HBV) – + Hepatitis D virus (HDV) – + Hepatitis A virus (HAV) – + Parvovirus B19 – + Herpes Zoster Virus (VZV) – + Herpes simplex virus 1 – + Herpes simplex virus 2 – + Human herpes virus 6 – + Human herpes virus 8 – + West Nile Virus (WNV) – + Tick-borne encephalitis virus (TBEV) – + Adenovirus type 2 – + Adenovirus type 4 – + Adenovirus type 7 – + Rotavirus A – + Escherichia coli (E.coli) – + Staphylococcus aureus – + Streptococcus pyogenes – + Streptococcus agalactiae – + ROBUSTNESS The robustness of the assay is a measure of its capacity to remain unaffected by small and accidental variations in method parameters and provides an indication of its reliability during normal usage. As HIV Real-TM Quant Dx kit contains RT-PCR REAGENTS PACK 96 vials with ready-to-use lyophilized reagents, the only probable variation during normal usage of the kit could be the different volume of added eluted samples obtained from RNA purification step. The robustness of HIV Real-TM Quant Dx kit was evaluated adding different volume of eluted sample (from 46 to 52 µl). 20 HIV RNA negative plasma pools spiked with HIV RNA to a final concentration of 3 times 95 per cent cut-off value were added with small variations (from 46 to 52 µl) to the vials containing lyophilized reagents. All tested samples were found positive for HIV RNA. CROSS CONTAMINATION The cross contamination of the HIV Real-TM Quant Dx kit was evaluated for the manual sample preparation using RotorGene Q instrument. To demonstrate absence of cross contamination a panel of 20 samples consisting of alternate samples of negative plasma spiked with high concentration of HIV and negative plasma samples was tested. All the positive samples were correctly detected and gave a fluorescence positive reaction with good and clear sigmoid-shaped fluorescence curves and a Cycle threshold (Ct) less than 35 both in HIV channel and in Internal Control channel. All the negative samples gave no fluorescence signal in the HIV channel and the Internal Control channel showing no cross contamination at all. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 15 PROTOCOL FOR ROTORGENE™ 6000/Q 1. Switch on instrument by pressing the main switch, start Rotor-Gene software 2. Start a New Run Figure 1. Start “New Run” 3. Select the appropriate rotor, check the “Locking Ring Attached” box and click “Next”. Figure 2. Rotor and Locking Ring 4. Input operator’s name, notes, and reaction volume (50). Subsequently press “Next”. Figure 3. Reaction Volume Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 16 5. Click the “Edit Profile” and program the temperature profile. Figure 4. Edit Profile Figure 5. Hold: Reverse transcription step 1 2 Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 17 Figure 6. Hold 2: Enzyme activation step Figure 7. Cycling: Amplification Step Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 18 Click “Gain Optimisation” in the “New Run Wizard” menu and set the following parameters: Figure 8. Gain Optimisation 6. Place PCR tubes carefully onto RotorGene™ 6000/Q rotor, close lid and start run. Figure 9. Starting the run. 7. Enter sample positions and names (“unknown” for samples, “Negative Control” and “Positive Control” for controls). If the kit calibration run is performing, use name “standard” for CAL1 and CAL2 and enter the concentrations of the Quantitative Standards reported on HIV Real-TM Quant Dx Data Card. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 19 8. User must import the calibration experiment with Standard Curves to subsequent experiments with clinical samples to have quantitative results. To do this click Import Curve, select From Other Run option, choose the Calibration file to be imported and Import ROX/Orange Channel. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 20 Data Analysis IC amplification analysis (Cycling A.Green (Fam) 1. Press Analysis and then select Quantitation Cycling A.Green Show 2. Turn off the automatic option Threshold. 3. Activate the Dynamic tube and Slope Correct buttons in the main window menu (Quantitation analysis). 4. In the table of results (Quantitation Analysis) select More settings and set NTC Threshold to 10% 5. Select Threshold: 0,05 and Eliminate Cycles before: 5. 6. In the table of results (Quantitation Results) appear the values of Ct (Threshold cycle) which should be 30 (see Internal Control) of the IC (Fam/Green channel). HIV amplification analysis (Cycling A.Orange (Rox) 1. Press Analysis and then select Quantitation Cycling A.Orange Show Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 21 2. Turn off the automatic option Threshold. 3. Press the buttons Dynamic Tube, Slope Correct 4. In the table of results (Quantitation Analysis) select More settings and set NTC Threshold to 10%. 7. Select Threshold: 0,05 and Eliminate Cycles before: 5. 5. In the table of results (Quantitation Analysis) appear the values of Ct (Threshold cycle). 6. In the new window Quantitation Results appear values of HIV RNA IU/ml. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 22 PROTOCOL FOR SaCycler-96® Real Time PCR system Recommended settings: 1. Double click on the RealTime_PCR software icon 2. Select Device handling. 3. Select the plugged device and click “Connect”. 4. Select from Menu “Test -> Create/Edit test” . Click “Create new test” button and enter name “HIV Real-TM Quant DX”, click OK. 5. Select: Analysis Type “Multiplex detection” and Method “Threshold”, Standard Quantity “2” and copy “3” (total 6 standards), 2 positive controls, 1 negative control, FAM (IC) and ROX (s), 50 µl of Mixture volume: 6. Under “Amplification Program”, click to create a new program. 7. Select “Preliminary heating” and choose the template below. Click Apply, then program the instrument as follows. 8. Click and save the amplification program file in a convenient folder. Then click OK in the previous window. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 23 9. Click add test button , then select “HIV Real-TM Quant DX” from dropdown menu Test, indicate the number of samples and click first Add, then OK. 10. Use the default Autofill positioning of PCR tubes into the plate or the Free Filling to chose manually the position of the tubes. In the “Concentration” column, click the orange circle and insert the HIV standard values reported in the Data Card (see example below). 11. Clicking Apply will bring to the Start Program window . 12. The thermalcycling program will be the following: 13. Click Open block, insert PCR tubes into the instrument, click Close block and then click to start the PCR amplification program. 14. After the run is complete click OK to interpret the results. 15. Select analysis type “Multiplex detection”, “Threshold (Ct)” method and “Quantitative” from results table. 16. Adjust the threshold line using mouse drag and drop. Suggested values for Threshold line are: 40 for ROX channel, 25 for FAM channel (slightly adapt threshold level according to your result curves). 17. Viral load results will appear in the “Results” column for ROX channel. Final results are already expressed in IU/ml. 18. To import standard curve from other run, click on “Additional standards” tab and select the run file containing standards. Then come back to “Results” tab to see viral load results. NOTE: for IVD version of SaCycler, since the HIV Real-TM DX protocol is already inserted in the PC supplied with the instrument, ignore points 4-8 and just follow instructions from point 9. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 24 TROUBLESHOOTING 1. Weak (Ct > 30) or exceeding the established range signal (see Internal Control) of the IC (Fam/Green channel): retesting of the sample is required. The PCR was inhibited. Make sure that you use a recommended RNA extraction method and follow the manufacturer’s instructions. The reagents storage conditions didn’t comply with the instructions. Check the storage conditions The PCR conditions didn’t comply with the instructions. Check the PCR conditions and for the IC detection select the fluorescence channel reported in the protocol. The IC was not added to the sample during the pipetting of reagents. Make attention during the RNA extraction procedure. 2. The correlation coefficient R2 is less than 0.95: retesting of all samples is required. 3. The calculated concentration of CONTROL + is different from given control concentration, reported in the Data Card: retesting of all samples is required. 4. Any signal on the ROX/Orange channel with Negative Control of extraction. Contamination during RNA extraction procedure. All samples results are invalid. Decontaminate all surfaces and instruments with sodium hypochlorite and ethanol. Use only filter tips during the extraction procedure. Change tips among tubes. Repeat the RNA extraction with the new set of reagents. 5. Any signal with Negative PCR Control. Contamination during PCR preparation procedure. All samples results are invalid. Decontaminate all surfaces and instruments with sodium hypochlorite and ethanol or special DNA decontamination reagents. Pipette the Positive controls at the end. Repeat the PCR preparation with the new set of reagents. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 25 REFERENCES 1. Sherman GG, Lilian RR, Bhardwaj S, Candy S, Barron P. – Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. – S Afr Med J. 2014 Mar;104(3 Suppl 1):235-8. 2. Swaminathan S, Hu Z, Rupert AW, Higgins JM, Dewar RL, Stevens R, Chen Q, Rehm CA, Metcalf JA, Baseler MW, Lane HC,Imamichi T. – Plasma Interleukin-27 (IL-27) Levels Are Not Modulated in Patients with Chronic HIV-1 Infection. – PLoS One. 2014 Jun 4;9(6):e98989. doi: 10.1371/journal.pone.0098989. eCollection 2014. 3. Shtarkshall RA. – An Interactive-Integrative Approach to Educational Interventions Aimed at Reducing HIV Infection. – Int J Adolesc Med Health. 2011 May 18;4(3-4):251-64. doi: 10.1515/IJAMH.1989.4.3-4.251. 4. Pantano S, Tyagi M, Giacca M, Carloni P. – Molecular dynamics simulations on HIV-1 Tat. – Eur Biophys J. 2004 Jul;33(4):344-51. Epub 2003 Nov 8. 5. Bahbouhi B, Landay A, Al-Harthi L – Dynamics of cytokine expression in HIV productively infected primary CD4+ T cells. – Blood. 2004 Jun 15;103(12):4581-7. Epub 2004 Feb 5. 6. Ferreira V, Moura P, Crovella S, Sobhie Diaz R, Castelo Filho A, Ximenes R, Arraes LC. – The Influence of HIV-1 Subtype in the Response to Therapeutic Dendritic Cell Vaccine. – Open AIDS J. 2012;6:289-92. doi: 10.2174/1874613601206010289. Epub 2012 Dec 14. 7. Cardona-Arias JA, Higuita-Gutiérrez LF – Impact of HIV/AIDS on quality of life: meta-analysis 2002-2012 – Rev Esp Salud Publica. 2014 Jan-Feb;88(1):87-101. doi: 10.4321/S1135- 57272014000100006. 8. Kann RK, Seddon JM, Kyaw-Tanner MT, Henning J, Meers J. – Association between feline immunodeficiency virus (FIV) plasma viral RNA load, concentration of acute phase proteins and disease severity. – Vet J. 2014 Feb 6. pii: S1090-0233(14)00048-3. doi: 10.1016/j.tvjl.2014.01.023. 9. Chandrashekar S. – Half a decade of mini-pool nucleic acid testing: Cost-effective way for improving blood safety in India. – Asian J Transfus Sci. 2014 Jan;8(1):35-8. doi: 10.4103/0973- 6247.126688. 10. Kaur P, Khong WX, Wee SY, Tan EL, Pipper J, Koay E, Ng KY, Yap JK, Chew KK, Tan MT, Leo YS, Inoue M, Ng OT – Clinical evaluation of a low cost, in-house developed real-time RT-PCR human immunodeficiency virus type 1 (HIV-1) quantitation assay for HIV-1 infected patients. – PLoS One. 2014 Mar 6;9(3):e89826. doi: 10.1371/journal.pone.0089826. eCollection 2014. Sacace™ HIV Real-TM Quant Dx NEW VER 24.12.2020 26 * Rotor-Gene™ Technology is a registered trademark of Qiagen * SaCycler™ is a registered trademark of Sacace Biotechnologies SacaceBiotechnologiesSrl via Scalabrini, 44 – 22100 –Como – Italy Tel +390314892927 Fax +390314892926 mail: info@sacace.com web: www.sacace.com
حمل و نقل کالا
HPV genotypes 14 High Risk Quant Real-TM , sacace-100 t
امتیاز 0 از 5
درخواست محصول
.فقط مشتریانی که این محصول را خریداری کرده اند و وارد سیستم شده اند میتوانند برای این محصول دیدگاه(نظر) ارسال کنند.
محصولات پیشنهادی
SinaPure ONE(Simultaneous DNA and RNA extraction) 50React-EX6051
امتیاز 0 از 5
Sinapure-DNA (Cell culture, Tissues, Gram negative Bacteria and CSF)50t – PR881613-EX6011
امتیاز 0 از 5






نقد و بررسیها
هنوز بررسیای ثبت نشده است.