ناموجود
بازگشت به محصولات
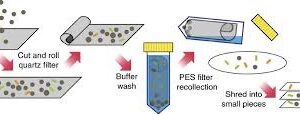

PsPure Genomic DNA from different sample-sacace-50t
2,160,000 تومان 1,800,000 تومان
SP HLA-B 27 Kit,sacace-24t
Sacace™ HLA-B27 Real-TM VER 26.09.2019 IVD For in Vitro Diagnostic Use HLA-B27 Real-TM Handbook Real Time PCR kit for detection of HLA-B27 polymorphism REF R116-50FRT 48 Sacace™ HLA-B27 Real-TM VER 26.09.2019 NAME HLA-B27 Real-TM INTRODUCTION Human Leukocyte Antigen (HLA) B27 (subtypes B*2701-2759) is a class I surface antigen encoded by the B locus in the major histocompatibility complex (MHC) on chromosome 6 and presents antigenic peptides (derived from self and non-self antigens) to T cells. HLA-B27 is strongly associated with ankylosing spondylitis (AS), and other associated inflammatory diseases referred to collectively as “spondyloarthritis”. The first human leukocyte antigen (HLA) haplotype association with inflammatory disease was discovered in 1972, correlating HLA-B27 with ankylosing spondylitis. This remains one of the strongest known associations of disease with HLA-B27. Since then, more than 100 disease associations have been made, including many ocular diseases and systemic diseases with specific ocular manifestations. According to the data published in the international ImMunoGeneTics database, 45 subtypes of HLA-B27 have been detected. A strong association has been found between the subtypes B*2705, B*2704,B*2702, and B*2707 and AS, whereas other subtypes are not associated with AS. Various methods have been developed for the identification of the HLA-B27 allele. The PCRbased HLA-typing methods, including the standard PCR with sequence-specific primers (SSP), have become widely used alternatives to serologic methods in clinical practice; however, these techniques are time-consuming and laborious. The drawbacks of the current methods, such as long processing time and the requirement for post-PCR manual procedures, have been overcome by the introduction of real-time PCR. INTENDED USE HLA-B27 Real-TM is a Real-Time amplification test for the detection of HLA-B27 in the biological materials. HLA-B27 Real-TM kit is designed to assist in the diagnosis of patients with suspected ankylosing spondylitis (AS) and other autoimmune diseases. The test is not intended for tissue typing. PRINCIPLE OF ASSAY HLA-B27 Real-TM Test is based on two major processes: isolation of genomic DNA from specimens and Real Time amplification with allele specific primers. The real-time PCR monitoring of fluorescence intensities allows the accumulating product detection without reopening of reaction tubes after the PCR run. HLA-B27 Real-TM PCR kit is a qualitative test Sacace™ HLA-B27 Real-TM VER 26.09.2019 which contains the Internal Control IC (beta-globine gene), which allows to control the presence of cellular material in the sample. MATERIALS PROVIDED “HLA-B27 Real-TM”: Real Time amplification kit PCR-mix, 0,96 ml PCR-buffer, 0,5 ml Taq-AT-polymerase, 0,025 ml TE-buffer, 1,2 ml Pos C+ (HLA B27& human DNA), 0,075 ml Contains reagents for 48 reactions MATERIALS REQUIRED BUT NOT PROVIDED DNA isolation kit Desktop microcentrifuge for “eppendorf” type tubes Vortex mixer Disposable gloves, powderless Biohazard waste container Refrigerator, Freezer Real Time Thermal cycler Pipettes (adjustable) Sterile pipette tips with filters STORAGE INSTRUCTIONS The whole kit HLA-B27 Real-TM must be stored at 2-8°C with the only exception of Taq-ATpolymerase that must be stored at -20°C. The kit can be shipped at 2-8°C for 3-4 days but the parts should be stored at 2-8°C and -20°C immediately on receipt. STABILITY HLA-B27 Real-TM is stable up to the expiration date indicated on the kit label. The product will maintain performance through the control date printed on the label. Exposure to light, heat or humidity may affect the shelf life of some of the kit components and should be avoided. Repeated thawing and freezing of these reagents should be avoided, as this may reduce the sensitivity. Sacace™ HLA-B27 Real-TM VER 26.09.2019 QUALITY CONTROL In accordance with Sacace’s ISO 13485-Certified Quality Management System, each lot is tested against predetermined specifications to ensure consistent product quality. WARNINGS AND PRECAUTIONS In Vitro Diagnostic Medical Device For In Vitro Diagnostic Use Only The user should always pay attention to the following: Use sterile pipette tips with aerosol barriers and use new tip for every procedure. Store extracted positive material (samples, controls and amplicons) away from all other reagents and add it to the reaction mix in a separate area. Thaw all components thoroughly at room temperature before starting an assay. When thawed, mix the components and centrifuge briefly. Use disposable gloves, laboratory coats and eye protection when handling specimens and reagents. Thoroughly wash hands afterwards. Do not eat, drink, smoke, apply cosmetics, or handle contact lenses in laboratory work areas. Do not use a kit after its expiration date. Dispose of all specimens and unused reagents in accordance with local authorities’ regulations. Specimens should be considered potentially infectious and handled in a biological cabinet in accordance with appropriate biosafety practices. Clean and disinfect all sample or reagent spills using a disinfectant such as 0.5% sodium hypochlorite, or other suitable disinfectant. Avoid sample or reagent contact with the skin, eyes, and mucous membranes. If skin, eyes, or mucous membranes come into contact, rinse immediately with water and seek medical advice immediately. Material Safety Data Sheets (MSDS) are available on request. Use of this product should be limited to personnel trained in the techniques of DNA amplification. The laboratory process must be one-directional, it should begin in the Extraction Area and then move to the Amplification and Detection Areas. Do not return samples, equipment and reagents to the area in which the previous step was performed. Some components of this kit contain sodium azide as a preservative. Do not use metal tubing for reagent transfer. Sacace™ HLA-B27 Real-TM VER 26.09.2019 PRODUCT USE LIMITATIONS Use of this product should be limited to personnel trained in the techniques of DNA amplification (UNI EN ISO 18113-2:2012). Strict compliance with the user manual is required for optimal PCR results. Attention should be paid to expiration dates printed on the box and labels of all components. Do not use a kit after its expiration date. SAMPLE COLLECTION, STORAGE AND TRANSPORT HLA-B27 Real-TM can analyze genomic DNA extracted from: whole blood collected in EDTA tubes. Specimens can be stored at +2-8°C for no longer than 48 hours, or freeze at -20°C to -80°C. Transportation of clinical specimens must comply with country, federal, state and local regulations for the transport of etiologic agents. DNA ISOLATION Any commercial RNA/DNA isolation kit, if IVD-CE validated for the specimen types indicated herein at the “SAMPLE COLLECTION, STORAGE AND TRANSPORT” paragraph, could be used: Sacace Biotechnologies recommends to use the following kits: Genomic column DNA Express – spin column extraction kit (Sacace, REF K-1-1/E) QIAamp DNA Blood mini kit (Qiagen, 51104) DNA samples with concentration in range from 20 to 200 ng/μl could be analyzed. Sacace™ HLA-B27 Real-TM VER 26.09.2019 PROTOCOL (total PCR reaction vol 35 µl, for Cepheid SmartCycler instrument 25 µl): 1. Prepare the required quantity of reaction tubes for samples (N) and controls (N+2). 2. Prepare the mixture of PCR-buffer and Taq-AT-polymerase by adding into a new sterile tube 10*N µl of PCR-buffer and 0,5*N µl of Taq-AT-polymerase (for Cepheid SmartCycler instrument add 5*N µl of PCR-buffer and 0,5*N µl of Taq-AT-polymerase). Vortex and centrifuge for 2-3 sec. 3. Add to each reaction tube 10 µl of the mixture PCR-buffer and Taq-AT-polymerase (5 µl for Cepheid SmartCycler instrument). 4. Add 20 µl of PCR-mix to each reaction tube containing the mixture PCR-buffer and Taq-ATpolymerase (15 µl of PCR-mix for Cepheid SmartCycler instrument). 5. Add to each correspondent reaction tube 5 µl of extracted genomic DNA. Mix by pipetting. 6. Prepare for each session 2 controls: ● add 5 µl of TE-buffer to the tube labeled Amplification Negative Control; ● add 5 µl of Pos C+ to the tube labeled Amplification Positive Control; 7. Insert the tubes in the thermalcycler. Amplification Create a temperature profile on your Real-time instrument1 as follows: Stage Тemp, °С Time Fluorescence detection Cycle repeats Hold 80 30 s – 1 Hold 94 5 min – 1 Cycling 94 30 s – 5 64 30 s – Cycling 2 94 10 s – 40 64 30 s FAM(Green), JOE(Yellow)/ HEX/Cy3 1 For example SaCycler-96™ (Sacace), Rotor-Gene™ 3000/6000/Q (Corbett Research, Australia – Qiagen, Germany), iQ5™/iQ iCycler™, CFX™ (BioRad); Mx3000P/Mx3005P™ (Agilent), ABI® 7300/7500 Real Time PCR (Applied), SmartCycler® (Cepheid) . Sacace™ HLA-B27 Real-TM VER 26.09.2019 RESULTS ANALYSIS The results are interpreted by the device software through the presence of crossing of fluorescence curve with the threshold line. DNA HLA-B27 is detected on the FAM(Green) channel and IC on the JOE(Yellow)/HEX/Cy3 channel. Results are accepted as relevant if both positive and negative controls of amplification show results in compliance with the table below: Results for controls Control Stage for control Ct channel Joe (Yellow)/ HEX/Cy3 Ct channel Fam (Green) Interpretation TE-buffer Amplification Neg Neg Valid result Pos C+ Amplification Pos Pos Valid result For interpretation of results please refer to table below: Results interpretation Ct Fam Ct Hex ∆Ct (Ct Fam – Ct Joe/Hex/Cy3) Interpretation Ct defined Ct defined Less than 8 (positive) HLA-B27 detected Ct defined Ct defined More than 10 (negative) HLA-B27 not detected Ct undefined Ct defined – Ct defined Ct defined 8-10 Result uncertain * Ct defined Ct undefined – Ct ≥33,0 – *If the result is uncertain, the DNA extraction or/and sampling procedure must be repeated. PERFOMANCES Assessment of the analytical performance of the Sacace HLA-B27 REAL-TIME PCR Kit Result TP 61 FP 0 FN 0 TN 89 Analytical sensitivity (AS) AS=TP/(TP+FN)х100% with 95% confidence interval, wherein TP is true positive results obtained using the tested kit and reference methods, FN is false negative results in the case of a negative result obtained using the tested kit and a positive result obtained by reference methods. Sacace™ HLA-B27 Real-TM VER 26.09.2019 Analytical specificity (ASp) ASp=TN/(TN+FP)х100% with 95% confidence interval, wherein TN is true negative results obtained using the tested kit and reference methods, FP is false positive results in the case of a positive result obtained using the tested kit and negative result obtained by reference methods. Parameter Clinical trials Analytical sensitivity (95% CI) Analytical specificity (95% CI) Sample number N=150 HLA-B27 100% (94.1-100%) 100% (95.9-100%) Assessment of the diagnostic performance of the Sacace HLA-B27 REAL-TIME PCR Kit Result TP 50 FP 0 FN 11 TN 89 Diagnostic sensitivity (DS) DS=TP/(TP+FN)х100% with 95% confidence interval, wherein TP is true positive results obtained using the tested kit and reference methods, FN is false negative results in the case of a negative result obtained using the tested kit and a positive result obtained by reference methods. Diagnostic specificity (DSp) DSp=TN/(TN+FP)х100% with 95% confidence interval, wherein TN is true negative results obtained using the tested kit and reference methods, FP is false positive results in the case of a positive result obtained using the tested kit and negative result obtained by reference methods. Parameter Clinical trials Diagnostic sensitivity (95% CI) Diagnostic specificity (95% CI) Sample number N=150 HLA-B27 100% (92.9-100%) 89% (81.2-94.4%) Sacace™ HLA-B27 Real-TM VER 26.09.2019 TROUBLESHOOTING 1. Absent signal of the IC (Joe (Yellow)/HEX/Cy3 channel): retesting of the sample is required. The PCR was inhibited. Make sure that you use a recommended DNA extraction method and follow the manufacturer’s instructions. The reagents storage conditions didn’t comply with the instructions. Check the storage conditions The PCR conditions didn’t comply with the instructions. Check the PCR conditions and for the IC detection select the fluorescence channel reported in the protocol. No correct sample collection or preparation. 2. No signal on the Joe (Yellow)/Cy3/HEX and Fam (Green) channels with Positive Control. The reagents storage conditions didn’t comply with the instructions. Check the storage conditions The PCR conditions didn’t comply with the instructions. Check the temperature profile and select the fluorescence channel reported in the protocol. Incorrect configuration of the PCR reaction: Check the reagents preparation step. 3. Any signal with Negative Control. Contamination during PCR preparation procedure. All samples results are invalid. Decontaminate all surfaces and instruments with sodium hypochlorite and ethanol or special DNA decontamination reagents. Pipette the Positive controls at the end. Repeat the PCR preparation with the new set of reagents. 4. Variation of 8-10 cycles between Ct values of Fam(Green) and Joe (Yellow) in a sample. Sample result is uncertain, retesting of sample is required. Sacace™ HLA-B27 Real-TM VER 26.09.2019 KEY TO SYMBOLS USED *SaCycler-96 is a registered trademark of Sacace Biotechnologies *iQ5™, CFX™ are registered trademarks of Bio-Rad Laboratories * Rotor-Gene™ Technology is a registered trademark of Qiagen * MX3005P® is a registered trademark of Agilent Technologies *ABI® is a registered trademark of Applied Biosystems *SmartCycler® is a registered trademark of Cepheid Sacace Biotechnologies Srl via Scalabrini, 44 – 22100 – Como – Italy Tel +390314892927 Fax +390314892926 mail: info@sacace.com web: www.sacace.com List Number Caution! Lot Number Contains sufficient for tests Expiration Date Version Store at NCA Negative Control of Amplification Manufacturer NCE Negative control of Extraction Consult instructions for use C+ Positive Control of Amplification IC Internal Control For in Vitro Diagnostic Use
حمل و نقل کالا
درخواست محصول
.فقط مشتریانی که این محصول را خریداری کرده اند و وارد سیستم شده اند میتوانند برای این محصول دیدگاه(نظر) ارسال کنند.
محصولات پیشنهادی
IPTG (Isopropyl-beta-D-thiogalactopyranoside) 5g-CL5812
امتیاز 0 از 5





نقد و بررسیها
هنوز بررسیای ثبت نشده است.