ناموجود
بازگشت به محصولات

JCV/BKV Virus Quant Real-TM ,sacace-100t
43,438,265 تومان 36,198,554 تومان
CMV/EBV/HHV6 Quant Real-TM ,sacace -100t
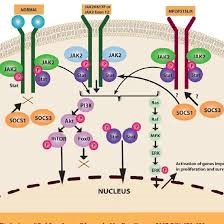
Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 For inVitro Diagnostic Use For Professional Use Only CMV/EBV/HHV6 Quant Real-TM Handbook Multiplex Real Time PCR Kit for quantitative detection and differentiation of Cytomegalovirus (CMV), Epstein Barr Virus (EBV) and Human Herpes 6 Virus (HHV6) REF V48-100FRT 100 Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 NAME CMV/EBV/HHV6 Quant Real-TM INTENDED USE The CMV/EBV/HHV6 Quant Real-TM PCR kit is an in vitro nucleic acid amplification test for qualitative and quantitative detection of human cytomegalovirus (CMV) DNA, Epstein-Barr virus (EBV) DNA and Human Herpes virus type 6 (HHV6) DNA in clinical material (whole blood, white blood cells, viscera biopsy material and cerebrospinal fluid) using real-time hybridization-fluorescence detection of amplified products. The results of PCR analysis are taken into account in complex diagnostics of disease. PRINCIPLE OF PCR DETECTION CMV, EBV and HHV6 detection by polymerase chain reaction (PCR) with hybridizationfluorescence detection includes DNA extraction from clinical samples and PCR amplification of pathogen genome specific region with real-time hybridizationfluorescence detection. During DNA extraction from clinical material, human genomic DNA (endogenous internal control) is amplified. Endogenous internal control (IC Glob) allows controlling both PCR-analysis stages (DNA extraction and PCR amplification), material sampling, and storage adequacy. Then, the obtained samples are amplified using specific primers and polymerase (TaqF). In real-time PCR, the amplified product is detected using fluorescent dyes. These dyes are linked to oligonucleotide probes which bind specifically to the amplified product during thermocycling. The real-time monitoring of the fluorescence intensities during the real-time PCR allows the detection of accumulating product without re-opening the reaction tubes after the PCR run. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 MATERIALS PROVIDED Reagent Description Volume, ml Quantity PCR-mix-1-FRT EBV / CMV / HHV-6 / Glob colorless clear liquid 0.6 2 tubes PCR-mix-2-FRT colorless clear liquid 0.3 2 tubes Polymerase (TaqF) colorless clear liquid 0.03 2 tubes RNA-buffer colorless clear liquid 0.6 1 tube DNA calibrator KSG1 colorless clear liquid 0.2 1 tube DNA calibrator KSG2 colorless clear liquid 0.2 1 tube Negative Control (C-) * colorless clear liquid 1.2 2 tubes Positive Control DNA EBV / CMV / HHV-6 and human DNA ** colorless clear liquid 0.1 2 tubes Contains reagents for 110 tests. * must be used in the extraction procedure as Negative Control of Extraction. ** must be used in the extraction procedure as Positive Control of Extraction (PCE). MATERIALS REQUIRED BUT NOT PROVIDED • DNA extraction kit. • Transport medium. • Disposable powder-free gloves. • Pipettes (adjustable). • Sterile pipette tips with aerosol barriers. • Disposable polypropylene 1,5/2,0 ml tubes. • Tube racks. • Vortex mixer. • Desktop centrifuge with rotor for 1,5/2,0 ml tubes. • PCR Workstation. • Real Time Thermal cycler. • Disposable polypropylene microtubes for PCR. • Refrigerator for 2–8 °C. • Deep-freezer for ≤ –16 °C. • Waste bin for used tips. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 QUALITY CONTROL In accordance with Sacace’s ISO 13485-Certified Quality Management System, each lot is tested against predetermined specifications to ensure consistent product quality. PRODUCT USE LIMITATIONS All reagents may exclusively be used in in vitro diagnostics. Use of this product should be limited to personnel trained in the techniques of DNA amplification (UNI EN ISO 18113-2:2012). Strict compliance with the user manual is required for optimal PCR results. Attention should be paid to expiration dates printed on the box and labels of all components. Do not use a kit after its expiration date. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 WARNINGS AND PRECAUTIONS In Vitro Diagnostic Medical Device For In Vitro Diagnostic Use Only 1. Wear disposable gloves, laboratory coats and eye protection when handling specimens and reagents. Thoroughly wash hands afterward. 2. Do not pipette by mouth. 3. Do not eat, drink, smoke, apply cosmetics, or handle contact lenses in laboratory work areas. 4. Do not use a kit after its expiration date. 5. Dispose of all specimens and unused reagents in accordance with local regulations. 6. Biosafety Level 2 should be used for materials that contain or are suspected of containing infectious agents. 7. Clean and disinfect all spills of specimens or reagents using a disinfectant such as 0,5% sodium hypochlorite, or other suitable disinfectant. 8. Avoid contact of specimens and reagents with the skin, eyes and mucous membranes. If these solutions come into contact, rinse immediately with water and seek medical advice immediately. 9. Material Safety Data Sheets (MSDS) are available on request. 10.Use of this product should be limited to personnel trained in the techniques of DNA amplification. 11.PCR reactions are sensitive to contamination. Measures to reduce the risk of contamination in the laboratory include physically separating the activities involved in performing PCR in compliance with good laboratory practice. 12.Workflow in the laboratory must proceed in a uni-directional manner, beginning in the Extraction Area and moving to the PCR and Detection Area. Do not return samples, equipment and reagents in the area where you performed previous step. Some components of this kit contain sodium azide as a preservative. Do not use metal tubing for reagent transfer. Sampling of biological materials for PCR-analysis, transportation, and storage are described in details in the handbook of the manufacturer. It is recommended that this handbook is read before beginning of the work. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 STORAGE INSTRUCTIONS The components of CMV/EBV/HHV6 Quant Real-TM PCR kit must be stored at 2–8 ºC excepting Polymerase (TaqF), PCR-mix-1-FRT EBV/CMV7HHV-6/Glob and PCR-mix2-FRT that must be stored at -16°C or below. The kit can be shipped at 2-8°C for no longer than 5 days but should be stored at 2-8°C and -16°C or below immediately on receipt. STABILITY CMV/EBV/HHV6 Quant Real-TM is stable up to the expiration date indicated on the kit label. The product will maintain performance through the date printed on the label. Exposure to light, heat or humidity may affect the shelf life of some of the kit components and should be avoided. Repeated thawing and freezing of these reagents should be avoided, as this may reduce the sensitivity. The shelf life of reagents before and after the first use is the same, unless otherwise stated. SAMPLE COLLECTION, STORAGE AND TRANSPORT CMV/EBV/HHV6 Quant Real-TM can analyze DNA extracted from: • whole peripheral and umbilical cord blood collected in either ACD or EDTA tubes; • white blood cells (buffy coat); • biopsy material homogenized with mechanical homogenizer and dissolved in PBS sterile; • CSF (Liquor); It is recommended to process samples immediately after collection. Store samples at 2–8 °C for no longer than 24 hours, or freeze at –20/80°C. Transportation of clinical specimens must comply with country, federal, state and local regulations for the transport of etiologic agents. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 DNA ISOLATION Any commercial RNA/DNA isolation kit, if IVD-CE validated for the specimen types indicated herein at the “SAMPLE COLLECTION, STORAGE AND TRANSPORT” paragraph, could be used. Sacace Biotechnologies recommends to use the following kits: ⇒ DNA-Sorb-B (Sacace, REF K-1-1/B/100); ⇒ DNA/RNA-Prep (Sacace, REF K-2-9); Please carry out the DNA extraction according to the manufacturer’s instructions. REAGENTS PREPARATION (REACTION VOLUME 25 µL): The total reaction volume is 25 µl, the volume of DNA sample is 10 µl. 1. Prepare the required number of the tubes for amplification of DNA from clinical and control samples. 2. Prepare in a new sterile tube the Reaction Mix. For each sample mix 10*N µl of PCR-mix-1-FRT EBV/CMV7HHV-6/Glob, 5,0*N µl of PCR-mix-2-FRT and 0,5*N µl of Polymerase (TaqF). Vortex and centrifuge for 2-3 sec. 3. Add 15 µl of Reaction Mix and 10 µl of extracted DNA sample to appropriate tube. Mix by pipetting. 4. Carry out the control amplification reactions: For qualitative analysis: NCA – Add 10 µl of RNA-buffer to the tube labeled NCA (Negative Control of Amplification). C+ – Add 10 µl of DNA calibrator KSG2 to the tube labeled C+ (Positive Control of Amplification). For quantitative analysis: NCA – Add 10 µl of RNA-buffer to the tube labeled NCA (Negative Control of Amplification). Calibrators – Add 10 µl of DNA calibrator KSG1 into two tubes. – Add 10 µl of DNA calibrator KSG2 into two tubes. Close tubes and transfer them into the instrument in this order: samples, negative controls, positive control, calibrators. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 Amplification Create a temperature profile on your instrument as follows: Step Rotor type instruments1 Plate or modular type instruments2 Temperature, °С Time Cycles Temperature, °С Time Cycles Hold 95 15 min 1 95 15 min 1 Cycling 95 5 sec 5 95 5 sec 60 20 sec 60 20 sec 5 72 15 sec 72 15 sec Cycling 2 95 5 sec 40 95 5 sec 60 40 20 sec fluorescence detection 60 30 sec fluorescence detection 72 15 sec 72 15 sec 1 For example, Rotor-GeneTM 3000 / Rotor-GeneTM 6000 (Qiagen) or equivalent. 2 For example iCycler iQTM,/ iQ5TM(BioRad), Mx3000PTM (Stratagene) or equivalent. Fluorescence is detected at the 2nd step of Cycling 2 stage (60 °C) in FAM/Green, JOE/Yellow/HEX/Cy3, ROX/Orange/Texas Red, and Cy5/Red fluorescence channels. INSTRUMENT SETTINGS (Rotor-Gene 6000/Q) Rotor-type instruments Channel Calibrate/Gain Optimization… Threshold More Settings/ Outlier Removal Slope Correct FAM/Green from 5 Fl to 10 Fl 0.03 10 % On JOE/Yellow from 5 Fl to 10 Fl 0.03 10 % On Rox/Orange from 5 Fl to 10 Fl 0.03 10 % On Cy5/Red from 5 Fl to 10 Fl 0.03 10 % On Plate- or modular type instruments The threshold line should cross only sigmoid curves of signal accumulation of positive samples and should not cross the baseline; otherwise, the threshold level should be raised. Set the threshold at a level where fluorescence curves are linear and do not cross curves of the negative samples. DATA ANALYSIS • β-Globin gene DNA (IC) is detected in the FAM/Green channel, • EBV DNA is detected in the JOE/HEX/Cy3/Yellow channel, • CMV DNA is detected in the ROX/Texas Red/Orange channel, • HHV6 DNA is detected in the Cy5/Red channel. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 RESULTS INTERPRETATION The results are interpreted by the software of the used Instrument by the crossing (or not) of the fluorescence curve with the threshold line. 1. The sample is considered to be positive for EBV DNA if its Ct value in the results grid on the JOE/HEX/Cy3/Yellow channel is detected and does not exceed the boundary value. 2. The sample is considered to be positive for CMV DNA if its Ct value in the results grid on the ROX/Orange/Texas Red channel is defined and does not exceed the boundary value. 3. The sample is considered to be positive for HHV6 DNA if its Ct value in the results grid on the Cy5/Red channel is defined and does not exceed the boundary value. 4. For qualitative analysis, the sample is considered to be negative if its Ct value in the results grid in the FAM/Green channel does not exceed the Ct value indicated in the Boundary Ct values table. 5. For quantitative analysis, the quantity of IC Glob DNA should be greater than 2000 copies per reaction for whole blood, white blood cells, viscera biopsy material or more than 500 copies per reaction for saliva and oropharyngeal swabs. For cerebrospinal fluid (liquor), the Ct value can be greater than the Ct value indicated in the Boundary Ct values table in the results grid in the FAM/Green channel or the quantity of IC Glob DNA can be less than 500 copies per reaction in case of quantitative analysis because the cerebrospinal fluid samples may contain a very small number of cells. For qualitative analysis, the result of analysis is considered to be invalid if the Ct value is not detected in the results grid (the fluorescence curve does not cross the threshold line) or if it is greater than the threshold value in the JOE/HEX/Yellow, ROX/Orange, or Cy5/Red channel and the Ct value in the results grid in the FAM/Green channel exceeds the Ct value indicated in the Boundary Ct values table. For quantitative analysis, the analysis result is considered to be invalid if the Ct value is not detected in the results grid (the fluorescence curve does not cross the threshold line) or if it is greater than the boundary value in the JOE/Yellow/HEX, ROX/Orange, or Cy5/Red channel and the quantity of IC Glob DNA is less than 2000 copies per reaction for whole blood, white blood cells, viscera biopsy material or if it is less than 500 copies per reaction for saliva and oropharyngeal swabs. In such cases, PCR analysis of the sample should be repeated. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 For qualitative analysis, results of analysis are considered reliable only if the results obtained for both Positive and Negative Controls of amplification as well as for the Negative Control of extraction are correct. For quantitative analysis, results on C+ should fall in range of concentrations indicated in the Product Data Card. Results for controls Control Stage for control Expected result Interpretation FAM/Green JOE/HEX/ Cy3/Yellow ROX/Orange/ Texas Red Cy5/Red NCE DNA extraction, PCR Neg Neg Neg Neg OK NCA PCR Neg Neg Neg Neg OK C+ DNA extraction, PCR Pos Pos Pos Pos OK KSG1 KSG2 PCR Pos Pos Pos Pos OK Boundary Ct values for Rotor-type instruments Sample FAM/Green Joe/HEX/Yellow ROX/Orange Cy5/Red NCE Absent Absent Absent Absent NCA Absent Absent Absent Absent C+ < 30 < 30 < 30 < 30 KSG2 < 30 < 30 < 30 < 30 Clinical samples < 23 < 30 < 31 < 31 Boundary Ct values for Plate type instruments Sample FAM/Green Joe/HEX/Yellow ROX/Orange Cy5/Red NCE Absent Absent Absent Absent NCA Absent Absent Absent Absent C+ < 35 < 35 < 35 < 35 KSG2 < 35 < 35 < 35 < 35 Clinical samples < 28 < 35 < 36 < 36 Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 Quantitative results In quantitative analysis, if total DNA is extracted from human whole blood, white blood cells and biopsy material, the concentration in log of DNA copies per standard cell quantity (105) in control and test samples is calculated by the following formula: For CMV: log {CMV DNA copies in PCR sample x 2*105} = log {CMV DNA copies/105 of cells}. Glob DNA copies in PCR sample For EBV: log {EBV DNA copies in PCR sample x 2*105} = log {EBV DNA copies/105 of cells}. Glob DNA copies in PCR sample For HHV6: log {HHV6 DNA copies in PCR sample x 2*105} = log {HHV6 DNA copies/105 of cells}. Glob DNA copies in PCR sample If total DNA is extracted from saliva, oropharyngeal swabs and cerebrospinal fluid (liquor), the concentration of DNA per ml of sample (Conc DNA) is calculated by the following formula: Conc DNA = C DNA х 100 (copies/ml) C DNA is the number of EBV DNA copies, or the number of CMV DNA copies, or the number of HHV6 DNA copies in DNA sample. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 Example of Qualitative Analysis (plate-type instrument) QUALITY CONTROL PROCEDURE CMV/EBV/HHV6 Quant Real-TM PCR contains the Internal Control IC (human betaglobine gene), which allows to control the presence of cellular material in the sample. If the sample is not correctly prepared or it is an insufficient quantity of epithelial cells the Internal Control will not be detected. A negative control of extraction (NCE), negative amplification control (NCA), positive amplification control (C+) are required for every run to verify that the specimen preparation, the amplification and the detection steps are performed correctly. If the controls are out of their expected range (see table Results for Controls), all of the specimens and controls from that run must be processed beginning from the sample preparation step. Ct limits (for plate type instruments) IC EBV CMV HHV6 28 35 36 36 No. Desription Fam (IC) Joe (EBV) Rox (CMV) Cy5 (HHV6) Result EBV CMV HHV6 Name Ct Ct Ct Ct 1 344 27,18 28 HHV6 – – + 2 445 26,41 34,12 32,1 CMV, HHV6 – + + 3 451 29,81 Invalid ? ? ? 4 456 23,3 28,48 27,7 EBV, HHV6 + – + 5 461 29,02 35,08 Invalid-?, (low CMV) ? low ? 6 472 24,83 33,28 EBV + – – 7 477 17,51 24,06 34,95 EBV, HHV6 + – + 8 489 21,32 21,85 27,2 EBV, HHV6 + – + 9 491 23,47 28,15 EBV + – – 10 494 29,88 Invalid ? ? ? 11 497 16,29 31,06 34,18 EBV, HHV6 + – + 12 501 18,5 32,64 CMV – + – 13 C+ 27,23 30,18 28,47 27,25 OK 14 C+ 26,06 30,45 27,95 26,58 OK 15 C+ 26,37 30,8 28,17 26,73 OK 16 C- (Neg. Control) OK 17 C- (DNA-buffer) OK 18 C- (DNA-buffer) OK Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 PERFORMANCE CHARACTERISTICS Sensitivity The analytical sensitivity of CMV/EBV/HHV6 Quant Real-TM PCR kit is specified in the table below. Type of clinical material Nucleic acid extraction kit Sensitivity Cerebrospinal fluid (liquor) DNA/RNA-Prep 400 copies/ml Whole blood, white blood cells, viscera biopsy material DNA/RNA-Prep 5 DNA copies per 105 cells Specificity CMV/EBV/HHV6 Quant Real-TM PCR kit is intended for Epstein-Barr virus (EBV) DNA, Human Herpes Virus type 6 (HHV6) DNA and human cytomegalovirus (CMV) DNA detection. Specific activity of CMV/EBV/HHV6 Quant Real-TM PCR kit was confirmed by analysis of reference CMV strain AD 169, QCMD panel for Epstein-Barr virus, as well as by analysis of clinical material with subsequent confirmation of results by sequencing the amplified fragments. The activity of the PCR kit components with respect to DNA of other viruses (herpes simplex virus types 1 and 2, human herpes virus type 8, Varicella Zoster Virus, Parvovirus B19, and others), bacterial pathogens (Staphylococcus aureus, Streptococcus pyogenes, Streptococcus agalactiae, and others) and human DNA was absent. The clinical specificity of CMV/EBV/HHV6 Quant Real-TM PCR kit was confirmed in laboratory clinical trials. Target region Channel for fluorophore FAM JOE ROX Cy5 DNA-target IC Glob DNA EBV DNA CMV DNA HHV6 DNA Target gene β-globin gene LMP-gene exon 4 of MIE (major immediate early) gene DNA polymerase catalytic subunit Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 TROUBLESHOOTING Results of analysis are not taken into account in the following cases: 1. The presence of any Ct value on JOE/Yellow/HEX, FAM/Green, ROX/Orange and Cy5/Red channels in the results grid for the Negative Control of Amplification (NCA) and for the Neg. Control of Extraction (C-) indicates contamination of reagents or samples. In this case, PCR analysis should be repeated for all samples in which pathogen DNA was detected starting from the DNA extraction stage. 2. For qualitative analysis, if the Ct value in the results grid for the Positive Control of PCR on the JOE/Yellow/HEX, FAM/Green, ROX/Orange, or Cy5/Red channels is absent, it is necessary to repeat amplification for all samples where pathogen DNA was not detected. 3. If the Ct value for the sample is not detected on JOE/Yellow/HEX/Cy3, ROX/Orange/Texas Red, Cy5/Red channel or it exceeds the boundary Ct value specified in the Boundary Ct values table and the Ct value for the sample is greater than the maximum Ct value for IC in the FAM/Green channel, analysis should be repeated starting from the DNA extraction stage. This error may be caused by incorrect treatment of clinical material, which resulted in the loss of DNA, or by the presence of PCR inhibitors. 4. If the Ct value for the sample is detected in JOE/Yellow/HEX/Cy3, ROX/Orange/Texas Red or Cy5/Red channel and it is greater than the boundary Ct value specified in the Boundary Ct values table, the result is considered to be equivocal. It is necessary to repeat analysis of such sample in duplicate. If a reproducible positive Ct value is obtained, the result is considered to be positive; otherwise, the result is considered to be equivocal. Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 REFERENCES • PCR detection of cytomegalovirus DNA in serum as a diagnostic test for congenital cytomegalovirus infection.C T Nelson, A S Istas, M K Wilkerson, and G J Demmler. J ClinMicrobiol. 1995 December; 33(12): 3317–3318. • Detection of Cytomegalovirus DNA in Peripheral Blood of Patients Infected with Human Immunodeficiency Virus. D. Shibata, W. John Martin, Maria D. Appleman, Dennis M. Causey, J. M. Leedom, N.Arnheim. J Infect Dis. (1988) 158 (6): 1185-1192. • Multiplex PCR for six herpesviruses after hematopoietic stem cell transplantation. Sawada A, Koyama-Sato M, Yasui M, Kondo O, Ishihara T, Takeshita Y, Okamura T, Nishikawa M, Inoue M, KawaPediatr Int. 2011 Aug 2. doi: 10.1111/j.1442-200X.2011.03437. • Cytomegalovirus Infections in Non-immunocompromised and Immunocompromised Patients in the Intensive Care Unit.Florescu DF, Kalil AC. Infect Disord Drug Targets. 2011 Jun 16. • Comparison of PCR, Antigenemia Assay, and Rapid Blood Culture for Detection and Prevention of Cytomegalovirus Disease after Lung Transplantation. Adriana Weinberg, Tony N. Hodges, Shaobing Li, GuanyungCai, M. R. Zamora. Journal of Clinical Microbiology, February 2000, p. 768-772, Vol. 38, No. 2 • Optimization of Quantitative Detection of Cytomegalovirus DNA in Plasma by Real-Time PCR. Michael Boeckh,MeeiLi Huang, James Ferrenberg, Terry Stevens-Ayers, Laurence Stensland, W. Garrett Nichols, and Lawrence Corey. Journal of Clinical Microbiology, March 2004, p. 1142- 1148, Vol. 42, No. 3 • Quantification of Human Cytomegalovirus DNA by Real-Time PCR. ElyanneGault,Yanne Michel, AxelleDehée, ChahrazedBelabani, Jean-Claude Nicolas, Antoine Garbarg-Chenon. J ClinMicrobiol. 2001 February; 39(2): 772–775 • Definitions of Cytomegalovirus Infection and Disease in Transplant Recipients. Per Ljungman, Paul Griffiths, Carlos Paya, …Clin Infect Dis. (2002) 34 (8): 1094-1097 Sacace™ CMV/EBV/HHV6 Quant Real-TM VER 02.02.2022 KEY TO SYMBOLS USED * iCycler iQ™ / iQ5™ are registered trademarks of Bio-Rad Laboratories * Rotor-Gene™ is a registered trademark of Qiagen * MX3000P™ is a registered trademark of Agilent Technologies SacaceBiotechnologiesSrl via Scalabrini, 44 – 22100 –Como – Italy Tel +390314892927 mail: info@sacace.com web: www.sacace.com List Number Caution! Lot Number Contains sufficient for tests For in Vitro Diagnostic Use Version Store at NCA Negative Control of Amplification Manufacturer C– Negative control of Extraction Consult instructions for use C+ Positive Control of Amplification Expiration Date IC Internal Control
حمل و نقل کالا
HPV genotypes 14 High Risk Quant Real-TM , sacace-100 t
امتیاز 0 از 5
درخواست محصول
.فقط مشتریانی که این محصول را خریداری کرده اند و وارد سیستم شده اند میتوانند برای این محصول دیدگاه(نظر) ارسال کنند.
محصولات پیشنهادی
Cinna Clone-DNA Extraction Solution (DNG – plus) 20ml-EX6081
امتیاز 0 از 5
IPTG (Isopropyl-beta-D-thiogalactopyranoside) 5g-CL5812
امتیاز 0 از 5
Sinapure DNA (whole blood, serum and plasma)-PR881612-EX6001
امتیاز 0 از 5











نقد و بررسیها
هنوز بررسیای ثبت نشده است.