ناموجود
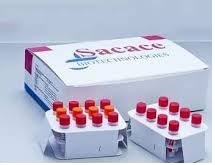
Dengue Real-TM (NEW),sacase -50t
IVD For in Vitro Diagnostic Use Dengue Real-TM Handbook Real Time PCR kit for qualitative detection of Dengue virus in clinical material REF V63-S-50FRT 50 Sacace™ Dengue Real-TM ver. 20.05.2016 Sacace™ Dengue Real-TM ver. 20.05.2016 NAME Dengue Real-TM INTRODUCTION Dengue is one of the most important arthropod-borne viral diseases with large global burden. The disease is caused by dengue virus (DENV), a member of Flaviviridae family. DENV is transmitted to human by Aedes mosquitoes as vector. Dengue clinical manifestations vary from asymptomatic or mild flu-like syndrome known as classic Dengue Fever (DF) to more severe form known as Dengue Hemorrhagic Fever (DHF) and the potentially fatal Dengue Shock Syndrome (DSS). Similar to other RNA viruses, DENV possess diverse genetic characteristics as shown by the presence of various genotypes within serotypes. Estimates of the global incidence of dengue infections per year have ranged between 50 million and 200 million; however, recent estimates using cartographic approaches suggest this number is closer to almost 400 million. The expansion of dengue is expected to increase due to factors such as the modern dynamics of climate change, globalization, travel, trade, socioeconomics, settlement and also viral evolution. No vaccine or specific antiviral therapy currently exists to address the growing threat of dengue. Acute infection with dengue virus is confirmed when the virus is isolated from serum or autopsy tissue specimens, or the specific dengue virus genome is identified by reverse transcriptionpolymerase chain reaction (RT–PCR) from serum or plasma, cerebrospinal fluid, or autopsy tissue specimens during an acute febrile illness. Methods such as one-step, real time RT–PCR are now widely used to detect dengue viral genes in acute-phase serum samples, approximately the first 5 days of symptoms. This detection coincides with the viremia and the febrile phase of illness onset. INTENDED USE DENGUE Real-TM PCR kit is an in vitro nucleic acid amplification test for qualitative detection of Dengue virus RNA in the biological material (blood plasma, histological (autopsy) material (brain and visceral tissues)) using real-time hybridization-fluorescence detection of amplified products. The PCR kit is used for studying the biological material, taken from the persons suspected of dengue fever without distinction of form and presence of manifestation. The results of PCR analysis are taken into account in complex diagnostics of disease. Sacace™ Dengue Real-TM ver. 20.05.2016 PRINCIPLE OF ASSAY Dengue virus detection by the polymerase chain reaction (PCR) is based on the RNA extraction form the test material with the Internal Control (IC) and simultaneous carrying out of reverse transcription reaction and amplification of Dengue virus cDNA fragments and Internal Control (IC) cDNA with hybridization-fluorescence detection. The Internal Control must be used in the extraction procedure in order to control the extraction process of each individual sample and to identify possible reaction inhibition. The RNA obtained at the RNA extraction stage is reverse transcribed by using the M-MLV Revertase, and amplified in to cDNA fragments by using specific primers and the enzyme Hot Start TaqF polymerase. In the real-time PCR, the amplified product is detected with the use of fluorescent dyes. These dyes are linked to oligonucleotide probes, which bind specifically to the amplified product during thermocycling. The real-time monitoring of fluorescence intensities during the real-time PCR allows the detection of accumulating product without re-opening the reaction tubes after the PCR run. DENGUE Real-TM PCR kit uses “hot-start”, which greatly reduces the frequency of nonspecifically primed reactions. “Hot-start” is guaranteed by the separation of nucleotides and TaqF polymerase by chemically modified polymerase (TaqF). The chemically modified polymerase (TaqF) is activated by heating at 95 °C for 15 min. At the RT-PCR stage, 2 reactions are carried out simultaneously in one tube – amplification of the Dengue virus cDNA and the Internal Control (IC) cDNA sequences. The amplification results of Dengue virus cDNA and Internal Control (IC) cDNA are detected in 2 different fluorescence detection channels: • the DENGUE virus cDNA is detected in the JOE/HEX/Yellow channel; • the IC cDNA is detected in the FAM/Green channel. Sacace™ Dengue Real-TM ver. 20.05.2016 MATERIALS PROVIDED Reagent Volume, ml Quantity PCR-mix-1-FRT DV 0.6 1 tube RT-PCR-mix-2-FRT 0.3 1 tube Hot Start TaqF Polymerase 0.03 1 tube M-MLV Revertase 0.015 1 tube RT-G-mix-2 0.015 1 tube Positive Control DV / IC (C+DV / IC) 0.2 1 tube RNA-buffer 0.2 1 tube Positive Control DV* 0.1 1 tube Internal Control (IC)** 0.5 1 tube Negative Control (C–)*** 1.2 7 tubes DENGUE Real-TM PCR kit is intended for 55 reactions (including controls). * must be used in the extraction procedure as Positive Control of Extraction: add 90 µl of C– (Negative Control) and 10 µl of Positive Control DV/IC (C+DV/IC) to the tube labeled Cpos; ** add 10 µl of Internal Control during the RNA extraction procedure of each sample directly to the sample/lysis mixture (see Ribo-Virus REF K-2/C/A or DNA/RNA-Prep REF K-2-9 protocol); *** must be used in the extraction procedure as Negative Control of Extraction: add 100 µl of C– (Negative Control) to the tube labeled Cneg. MATERIALS REQUIRED BUT NOT PROVIDED • Real Time Thermalcycler • RNA isolation kit • Desktop microcentrifuge for “eppendorf” type tubes • Vortex mixer • Disposable gloves, powderless • Biohazard waste container • Refrigerator, Freezer • Workstation • Pipettes (adjustable) • Sterile pipette tips with filters • Disposable polypropylene PCR tubes or strips • Tube racks Sacace™ Dengue Real-TM ver. 20.05.2016 STORAGE INSTRUCTIONS All components of the DENGUE Real-TM PCR kit are to be stored at or below minus 20ºC. They are stable until the expiration date indicated on the label. The kit can be shipped at 2-8°C but should be stored at -20°C immediately on receipt. STABILITY DENGUE Real-TM PCR kit is stable up to the expiration date indicated on the kit label. The product will maintain performance through the control date printed on the label. Exposure to light, heat or humidity may affect the shelf life of some of the kit components and should be avoided. Repeated thawing and freezing of these reagents should be avoided, as this may reduce the sensitivity. QUALITY CONTROL In accordance with Sacace’s ISO 13485-Certified Quality Management System, each lot is tested against predetermined specifications to ensure consistent product quality. Sacace™ Dengue Real-TM ver. 20.05.2016 WARNINGS AND PRECAUTIONS In Vitro Diagnostic Medical Device For In Vitro Diagnostic Use Only The user should always pay attention to the following: • Use sterile pipette tips with aerosol barriers and use new tip for every procedure. • Store extracted positive material (samples, controls and amplicons) away from all other reagents and add it to the reaction mix in a separate area. • Thaw all components thoroughly at room temperature before starting an assay. • When thawed, mix the components and centrifuge briefly. • Use disposable gloves, laboratory coats and eye protection when handling specimens and reagents. Thoroughly wash hands afterwards. • Do not eat, drink, smoke, apply cosmetics, or handle contact lenses in laboratory work areas. • Do not use a kit after its expiration date. • Dispose of all specimens and unused reagents in accordance with local authorities’ regulations. • Specimens should be considered potentially infectious and handled in a biological cabinet in accordance with appropriate biosafety practices. • Clean and disinfect all sample or reagent spills using a disinfectant such as 0.5% sodium hypochlorite, or other suitable disinfectant. • Avoid sample or reagent contact with the skin, eyes, and mucous membranes. If skin, eyes, or mucous membranes come into contact, rinse immediately with water and seek medical advice immediately. • Material Safety Data Sheets (MSDS) are available on request. • Use of this product should be limited to personnel trained in the techniques of DNA amplification. • The laboratory process must be one-directional, it should begin in the Extraction Area and then move to the Amplification and Detection Areas. Do not return samples, equipment and reagents to the area in which the previous step was performed. Some components of this kit contain sodium azide as a preservative. Do not use metal tubing for reagent transfer. PRODUCT USE LIMITATIONS All reagents may exclusively be used in in vitro diagnostics. Use of this product should be limited to personnel trained in the techniques of DNA amplification (UNI EN ISO 18113-2:2012). Strict compliance with the user manual is required for optimal PCR results. Attention should be paid to expiration dates printed on the box and labels of all components. Do not use a kit after its expiration date. Sacace™ Dengue Real-TM ver. 20.05.2016 SAMPLE COLLECTION, STORAGE AND TRANSPORT Note: Handle all specimens as if they are potentially infectious agents. Dengue Real-TM PCR kit is intended for analysis of RNA extracted with RNA extraction kits from the clinical/biological material blood plasma, histological autopsy material. • Blood plasma. Blood samples are taken after overnight fasting into a tube (special vacuum blood collection system) with EDTA as anticoagulant. The closed tube with blood is rotated several times for thoroughly mixing with the anticoagulant. It can be stored at 2–8 °С. The tubes with the whole blood are to be centrifuged no later than 6 hours from the blood collection at 800-1,600 g (for example, 3,500-5,000 rpm for the Eppendorf microcentrifuge) for 20 min at room temperature. No less than 1 ml of the obtained plasma is transferred into the sterile 2.0- ml tubes using a new filter tip for each sample. The blood plasma samples can be stored before the PCR analysis: – at the temperature from 2 to 8 °С for 1 day, – at the temperature from minus 24 to minus 16 °С – for 1 week, – at the temperature ≤ –70 °С – for a long time. Only one freeze-thawing cycle is required. • Histological (autopsy) material. Biopsy material is taken by a sterile tool (for example, tweezers) into a sterile plastic 50-ml container with tightly closed cap or 2 ml tube. The tube is to be closed tightly. The histological (autopsy) material samples can be stored: – at room temperature – for 6 hours, – at the temperature from 2 to 8 °С for 3 days, – at the temperature from minus 24 to minus 16 °С – for 1 week, – at the temperature ≤ –70 °С – for a long time. Pretreatment Blood plasma: The pretreatment is not required. Histological (autopsy) material: for RNA extraction 30-50 µl of the material is taken and homogenized by trituration using precooled sterile porcelain mortar and mallet or homogenizer. The suspension is prepared using grinded tissue and precooled sterile physiological solution or phosphate buffer. For this, 9 volumes of physiological solution are to be added to 1 volume of grinded tissue. Use 100 µl of suspension for RNA extraction. The pretreated autopsy material samples can be stored: – at the temperature from minus 24 to minus 16 °С – for 1 week, – at the temperature ≤ –70 °С – for a long time. Sacace™ Dengue Real-TM ver. 20.05.2016 Interfering substances and limitations of using test material samples In order to control the RNA extraction efficiency and possible reaction inhibition the Internal Control (IC) is used in the PCR kit. The Internal Control is added in each biological sample at the extraction stage. The presence of internal control signal after the amplification testifies the effectiveness of nucleic acid extraction and the absence of PCR inhibitors. The blood plasma samples collected into the tubes with heparin as anticoagulant are inapplicable for the analysis. Transportation of clinical specimens must comply with country, federal, state and local regulations for the transport of etiologic agents. RNA ISOLATION Any commercial RNA/DNA isolation kit, if IVD-CE validated for the specimen types indicated herein at the “SAMPLE COLLECTION, STORAGE AND TRANSPORT” paragraph, could be used. Sacace Biotechnologies recommends to use the following kits: Ribo-Virus – plasma (Sacace, REF K-2/C) DNA/RNA-Prep – plasma, histological material (Sacace, REF K-2-9) SaMag Viral Nucleic Acids Extraction kit – plasma (Sacace, REF SM003) Please carry out the RNA extraction according to the manufacturer’s instructions. The purified RNA can be stored at 2–8 °С for at most 4 h, at temperatures not higher than minus 16 °С for 1 month, and at temperatures not higher than minus 68 °С for one year. PROTOCOL: PREPARING TUBES FOR PCR The total reaction volume is 25 µl, the volume of the cDNA sample is 10 µl. 1. Before starting work, thaw and vortex all reagents of the kit. Make sure that there are no drops on the caps of the tubes. 2. Take the required number of PCR tubes for amplification of clinical and control samples (including the positive and negative controls of extraction and two controls of amplification). 3. To prepare the reaction mixture, mix in a new sterile tube the reagents per one reaction: – 10 µl of PCR-mix-FRT DV, – 5 µl of RT-PCR-mix-2-FRT, – 0.5 µl of Hot Start TaqF Polymerase, – 0.25 µl of RT-G-mix-2, – 0.25 µl of MMLV Revertase. Thoroughly vortex the mixture, make sure that there are no drops on the caps of the tubes. 4. Add 15 µl of the prepared reaction mixture to each PCR tube. Sacace™ Dengue Real-TM ver. 20.05.2016 5. Add 10 µl of RNA samples isolated from the clinical samples to each PCR tube. Mix the tubes thoroughly by pipetting avoiding foaming and make sure that there are no drops on the tube walls, otherwise vortex the tubes briefly. 6. Run the control reactions: C+ – Add 10 µl of the RNA sample extracted from the Positive Control to the tube labeled C+ (Positive Control of Extraction); C– – Add 10 µl of the RNA sample extracted from the Negative Control to the tube labeled C– (Negative Control of Extraction); C+ – Add 10 µl of Positive Control DV / IC (C+DV / IC) to the tube labeled C+ (Positive Control of Amplification); NCA – Add 10 µl of RNA-buffer to the tube labeled NCA (Negative Control of Amplification). 7. Insert the tubes into the device Amplification 1. Create a temperature profile on your instrument as follows: Step Temperature, °С Time Fluorescent signal detection Cycle repeats 1 50 15 min – 1 2 95 15 min – 1 3 95 10 s – 45 60 30 s FAM/Green, JOE/HEX/Yellow For example Rotor-Gene™ 6000/Q (Corbett Research, Qiagen), SaCycler-96™ (Sacace), CFX 96(BioRad) Fluorescent signal is detected in the FAM/Green and JOE/HEX/Yellow channels. INSTRUMENT SETTINGS Rotor-type instruments (RotorGene 6000, RotorGene Q) Channel Calibrate / Gain Optimisation Threshold More Settings/ Outlier Removal Slope Correct Eliminate Cycles before FAM/Green 5Fl – 10Fl 0,03 5% ON 5 JOE/Yellow 5Fl – 10Fl 0,03 5% ON 5 Plate- or modular type instruments (CFX™ (BioRad); SaCycler-96™ (Sacace), For result analysis, set the threshold line at a level where curves of fluorescence are linear. Sacace™ Dengue Real-TM ver. 20.05.2016 DATA ANALYSIS Analysis of results is performed by the software of the real-time RT-PCR instrument used by measuring fluorescence signal accumulation in 2 channels: – the IC cDNA is detected in the FAM/Green channel; – the DENGUE cDNA is detected in the JOE/HEX/Yellow channel. The results are interpreted by the real-time PCR instrument software by the crossing or not crossing of the threshold line by the fluorescence curve. The result of amplification is considered negative if the fluorescence curve is not S-shaped and if it does not cross the threshold line (the Ct value is absent). RESULTS INTERPRETATION Сt value in Result FAM JOE < boundary value absent Dengue RNA is not detected determined or absent < boundary value Dengue RNA is detected absent or > boundary value absent or > boundary value Invalid* < boundary value > boundary value Equivocal** * If the invalid result is obtained, PCR analysis is to be repeated for the corresponding test sample from the RNA extraction stage. ** If the equivocal result is obtained, PCR analysis is to be repeated for the corresponding test sample from the RNA extraction stage. If the same result is obtained the sample is considered as positive. If the negative result is obtained in the second run the sample is considered as equivocal. Resampling of biological material is recommended. Boundary Ct values Sample Channel FAM/Green JOE/Yellow C+ < 32 < 32 C– < 30 absent PCE < 30 < 32 NCA absent absent Test samples < 32 < 42 The result of the analysis is considered reliable only if the results obtained for Positive and Negative Controls of amplification as well as for the Positive and Negative Controls of extraction are correct: Control Stage for control Сt value in the channel for fluorophore FAM JOE PCE RNA extraction < boundary value < boundary value C– RNA extraction < boundary value Absent NCA RT-PCR Absent Absent C+ RT-PCR < boundary value < boundary value Sacace™ Dengue Real-TM ver. 20.05.2016 QUALITY CONTROL PROCEDURE A defined quantity of Internal Control (IC) is introduced into each sample and control at the beginning of sample preparation procedure in order to control the extraction process of each individual sample and to identify possible reaction inhibition. A positive control of extraction (PCE), negative control of extraction (NCE), negative amplification control (NCA), positive amplification control (C+) are required for every run to verify that the specimen preparation, the amplification and the detection steps are performed correctly. If the controls are out of their expected range (see table Results for Controls), all of the specimens and controls from that run must be processed beginning from the sample preparation step. SPECIFICATIONS Analitical Sensitivity The analytical sensitivity of DENGUE Real-TM PCR kit is specified in the table below. Test material Sample volume for extraction, µl Nucleic acid extraction kit Analytical sensitivity, copies/ml Blood plasma 100 DNA/RNA-prep 103 Histological (autopsy) material 100 5х103 Blood plasma 200 MAGNO-virus 103 1000 102 The claimed analytical features of DENGUE Real-TM PCR kit are guaranteed only when additional reagents kits “Magno-Virus”, or “DNA/RNA-prep” are used. Analitical Specificity The analytical specificity of DENGUE Real-TM PCR kit is ensured by the selection of specific primers and probes as well as stringent reaction conditions. The primers and probes have been checked for possible homologies to all sequences published in gene banks by sequence comparison analysis. The analytical specificity of DENGUE Real-TM PCR kit was proved while studying the following microorganisms’ strains: Flaviviridae: WNV (West Nile virus), JEV (Japanese encephalitis virus), OHFV (Omsk hemorrhagic fever virus), Tick-borne encephalitis virus (TBEV), LGT (Langat virus), POWV (Powassan virus), USUV (Usutu virus), and also CCHFV (Crimean-Congo hemorrhagic fever virus), СHV (chikungunya virus), Leptospira spp (strains MV5, HS, 3705), tick-borne rickettsiae of the spotted fever group (Rickettsia sibirica, Rickettsia conorii, Rickettsia raoultii, Rickettsia heilongjiangensis, Rickettsia slovaca, Rickettsia canadensis), A.phagocytophillum, B.microti, B.henselae, Y.pestis, and also the human genome DNA. Sacace™ Dengue Real-TM ver. 20.05.2016 The clinical specificity of DENGUE Real-TM PCR kit was confirmed in laboratory clinical trials. Target gene: 3’-UTR Reproducibility and repeatability The reproducibility and repeatability were determined by testing the model sample of biological material. The model sample of biological material was prepared by dilution of quality control sample containing Dengue virus RNA with concentration 5х102 copies/ml in the biological material. Test material Number of repeats Coefficient of variation (CV), % Spread in data within one test Blood plasma 8 0.6 Histological (autopsy) material 8 1.6 Spread in data between tests taken at different days Blood plasma 16 2.8 Histological (autopsy) material 16 2.0 Diagnostic characteristics The results of testing Dengue Real-TM PCR kit in comparison with the reference assay Samples type Results of using AmpliSens® Dengue virus-FRT PCR kit Results of using reference assay 1 Positive Negative Blood plasma 278 samples were tested Positive 96 0 Negative 2 180 Histological (autopsy) material 100 samples were tested Positive 100 0 Negative 0 0 The following samples were used: – 278 blood plasma samples obtained from the patients sought medical help with the dengue fever symptom; – 100 autopsy material samples. 4 of which was obtained from the died patient, 96 samples were obtained by addition of quality control sample containing Dengue virus RNA with concentration 5х103 copies/ml into the negative samples of viscera homogenates. 1 Reagents kits for determination antibodies of classes IgM and IgG against Dengue virus by enzyme immunoassay (EIA) Mosaic: Dengue virus types 1-4 IIFT (IgG), Mosaic: Dengue virus types 1-4 IIFT (IgM), (EUROIMMUN AG, Germany) were used as reference assay. The samples were considered as positive from the patients with the diagnosis confirmed by determination of seroconversion of specific antibodies. Sacace™ Dengue Real-TM ver. 20.05.2016 Diagnostic characteristics of the Dengue Real-TM PCR kit Samples type Diagnostic sensitivity 2, no less than % Diagnostic specificity 3 , no less than % Blood plasma 98 % 100 % Histological (autopsy) material 100 % 100 % TROUBLESHOOTING Results of analysis are not taken into account in the following cases: 1. The Ct value determined for the Positive Control of Amplification (C+) in the channel for the FAM and/or JOE fluorophore is greater than the boundary Ct value or absent. The amplification and detection should be repeated for all samples in which the specific RNA was not detected. 2. The Ct value determined for the Positive Control of Extraction (PCE) in the channel for the JOE fluorophore is greater than the boundary Ct value or absent. The PCR analysis (beginning with the RNA extraction stage) should be repeated for all samples. 3. The Ct value is determined for the Negative Control of Extraction (C–) in the channel for the JOE fluorophore. The contamination of laboratory with amplification fragments or contamination of reagents, test samples is probable at any stage of PCR analysis. Measures for detecting and elimination of contamination source must be taken. The PCR analysis (beginning with the RNA extraction stage) should be repeated for all samples in which the specific RNA was detected. 4. The Ct value is determined for the Negative Control of Amplification (NCA) in the channels for the FAM and/or JOE fluorophores. The contamination of laboratory with amplification fragments or contamination of reagents, test samples is probable at any stage of PCR analysis. Measures for detecting and elimination of contamination source must be taken. The amplification and detection should be repeated for all samples in which the specific RNA was detected. 5. The Ct value is determined for the test sample, whereas the area of typical exponential growth of fluorescence is absent (the graphic looks like approximate straight line). It is necessary to check the correctness of the threshold line level or parameters of base line calculation. If the result has been obtained with the correct threshold line (base line) level, the amplification and detection should be repeated for this sample. 2 Relative sensitivity in comparison with the used reference assay. 3 Relative specificity in comparison with the used reference assay. Sacace™ Dengue Real-TM ver. 20.05.2016 Sacace™ Dengue Real-TM ver. 20.05.2016 KEY TO SYMBOLS USED * SaCycler™ is a registered trademark of Sacace Biotechnologies * CFX™ are trademarks of Bio-Rad Laboratories * Rotor-Gene™ Technology is a registered trademark of Qiagen Sacace Biotechnologies Srl via Scalabrini, 44 – 22100 – Como – Italy Tel +390314892927 Fax +390314892926 mail: info@sacace.com web: www.sacace.com List Number Caution! Lot Number Contains sufficient for tests For in Vitro Diagnostic Use Version Store at NCA Negative Control of Amplification Manufacturer NCE Negative control of Extraction Consult instructions for use C+ Positive Control of Amplification Expiration Date IC Internal Control
حمل و نقل کالا
درخواست محصول
.فقط مشتریانی که این محصول را خریداری کرده اند و وارد سیستم شده اند میتوانند برای این محصول دیدگاه(نظر) ارسال کنند.
محصولات پیشنهادی









نقد و بررسیها
هنوز بررسیای ثبت نشده است.