ناموجود
بازگشت به محصولات

HBV Real-TM Quant DX Real-TM ,sacace,96 t
46,454,817 تومان 38,712,348 تومان
HPV Genotypes 6/11 Low Risk Quant Real-TM,sacace 100 t
Sacace™ HPV 6/11 Real-TM VER 05.11.2021 IVD For in Vitro Diagnostic Use HPV 6/11 Real-TM HANDBOOK Real Time PCR kit for qualitative detection of Human Papillomavirus 6 and 11 REF V11-100FRT 100 Sacace™ HPV 6/11 Real-TM VER 05.11.2021 NAME HPV 6/11 Real-TM INTRODUCTION Genital infection with HPV is one of the most common sexually transmitted diseases (STDs) of viral etiology worldwide (20% – 46% in different countries in sexually active young women). Cervical cancer is the second most common cancer in women worldwide, and a compelling body of clinical, epidemiological, molecular and experimental evidence has established the etiological relationship between some sexually transmitted HPV genotypes and cervical neoplasia throughout the world. Based on the frequency of detection of HPV genotypes from different grades of Cervical Intraepithelial Neoplasia (CIN Grades I – III), HPV genotypes are subdivided into High-risk HPV types (16, 18, 31 and 45), Intermediate-risk types (33, 35, 39, 51, 52, 56, 58, 59, and 68), and Low-risk types (6, 11, 42-44). Genital warts (technically known as condylomata acuminata) are most commonly associated with two HPV types, HPV 6 and HPV 11. Several methods have been used to diagnose clinical or subclinical infections with HPVs including clinical observation, cytological screening by Pap smear, electron microscopy, immunocytochemistry, but these methods have some disadvantages such as non-standardization and subjectivity, insufficient sensitivity and low predictable values. The PCR-based methods have been used successfully for the detection and typing of genital HPV genotypes in clinical specimens such as cervical swabs or scrapes, cervicovaginal lavages, frozen biopsies and formalin-fixed paraffin-embedded tissues. Recently many countries introduced quadrivalent HPV 6/11/16/18 Vaccine program therefore the detection of HPV 6 and 11 types can be used for the evaluation of efficiency of the profilaxis. INTENDED USE The kit HPV 6/11 Real-TM is an in vitro Real Time amplification test for qualitative detection of Human Papillomavirus 6 and 11 in the urogenital swabs. PRINCIPLE OF ASSAY The kit HPV 6/11 Real-TM is based on two major processes: isolation of DNA from specimens and Real Time amplification. PCR-mix-1 tube contains primers directed against regions of HPV types 6, 11 and - globine gene used as Internal Control. If the swab is not correctly prepared (high quantity of mucous or insufficient quantity of epithelial cells) the Internal Control will not be detected or will be low (the quantity of epithelial cells lower than 103 cells/reaction). HPV 6 is detected on the FAM (Green) channel, HPV 11 on the JOE (Yellow)/Cy3/HEX channel and Human -globine gene on the ROX (Orange)/Texas Red channel. Sacace™ HPV 6/11 Real-TM VER 05.11.2021 MATERIALS PROVIDED Real Time PCR kit (V11-100FRT) PCR-mix-1-FRT, 4 x 0,3 ml; PCR-Buffer-FRT, 2 x 0,3 ml; TaqF Polymerase, 2 x 0,03 ml; Pos Control Complex C+ (HPV 6, 11, human DNA), 0,2 ml; Negative Control C-, 1,2 ml;* DNA-buffer, 0,5 ml; Contains reagents for 120 tests. *must be used in the isolation procedure as Negative Control of Extraction. MATERIALS REQUIRED BUT NOT PROVIDED Disposable gloves, powderless Biohazard waste container Refrigerator, Freezer Real Time Thermal cycler Workstation Pipettes (adjustable) Sterile pipette tips with filters STORAGE INSTRUCTIONS HPV 6/11 Real-TM must be stored at -20°C. The kit can be shipped at 2-8°C but should be stored at – 20°C immediately on receipt. STABILITY HPV 6/11 Real-TM is stable up to the expiration date indicated on the kit label. The product will maintain performance through the control date printed on the label. Exposure to light, heat or humidity may affect the shelf life of some of the kit components and should be avoided. Repeated thawing and freezing of these reagents should be avoided, as this may reduce the sensitivity. QUALITY CONTROL In accordance with Sacace’s ISO 13485-Certified Quality Management System, each lot is tested against predetermined specifications to ensure consistent product quality. Sacace™ HPV 6/11 Real-TM VER 05.11.2021 WARNINGS AND PRECAUTIONS In Vitro Diagnostic Medical Device For In Vitro Diagnostic Use Only The user should always pay attention to the following: Use sterile pipette tips with aerosol barriers and use new tip for every procedure. Store extracted positive material (samples, controls and amplicons) away from all other reagents and add it to the reaction mix in a separate area. Thaw all components thoroughly at room temperature before starting an assay. When thawed, mix the components and centrifuge briefly. Use disposable gloves, laboratory coats and eye protection when handling specimens and reagents. Thoroughly wash hands afterwards. Do not eat, drink, smoke, apply cosmetics, or handle contact lenses in laboratory work areas. Do not use a kit after its expiration date. Dispose of all specimens and unused reagents in accordance with local authorities’ regulations. Specimens should be considered potentially infectious and handled in a biological cabinet in accordance with appropriate biosafety practices. Clean and disinfect all sample or reagent spills using a disinfectant such as 0.5% sodium hypochlorite, or other suitable disinfectant. Avoid sample or reagent contact with the skin, eyes, and mucous membranes. If skin, eyes, or mucous membranes come into contact, rinse immediately with water and seek medical advice immediately. Material Safety Data Sheets (MSDS) are available on request. Use of this product should be limited to personnel trained in the techniques of DNA amplification. The laboratory process must be one-directional, it should begin in the Extraction Area and then move to the Amplification and Detection Areas. Do not return samples, equipment and reagents to the area in which the previous step was performed. Some components of this kit contain sodium azide as a preservative. Do not use metal tubing for reagent transfer. PRODUCT USE LIMITATIONS All reagents may exclusively be used in in vitro diagnostics. Use of this product should be limited to personnel trained in the techniques of DNA amplification (UNI EN ISO 18113-2:2012). Strict compliance with the user manual is required for optimal PCR results. Attention should be paid to expiration dates printed on the box and labels of all components. Do not use a kit after its expiration date. Sacace™ HPV 6/11 Real-TM VER 05.11.2021 SAMPLE COLLECTION, STORAGE AND TRANSPORT HPV 6/11 Real-TM can analyze DNA extracted from: Cervical swabs: Remove excess mucus from the cervical os and surrounding ectocervix using a cotton or polyester swab. Discard this swab. Insert the Sampling Cervical Brush 1.0-1.5 centimeters into the cervical os until the largest bristles touch the ectocervix. Do not insert brush completely into the cervical canal. Rotate brush 3 full turns in a counterclockwise direction, remove from the canal. Insert brush into the nuclease-free 2,0 ml tube with 0,3 mL of Transport medium (Sacace). Vigorously agitate brush in medium for 15-20 sec. Snap off shaft at scored line, leaving brush end inside tube. Tissue homogenized with mechanical homogenizer and dissolved in PBS sterile (recommended DNA-Sorb-C REF K-1-6/50 not included in this kit, but can be ordered separately) Liquid-based cytology samples (Cytoscreen, PreservCyt) (recommended DNA-Sorb-D REF K-1- 8/100 not included in this kit, but can be ordered separately) It is recommended to process samples immediately after collection. Store samples at 2–8 °C for no longer than 24 hours, or freeze at –20/80°C. Transportation of clinical specimens must comply with country, federal, state and local regulations for the transport of etiologic agents. DNA ISOLATION Any commercial RNA/DNA isolation kit, if IVD-CE validated for the specimen types indicated herein at the “SAMPLE COLLECTION, STORAGE AND TRANSPORT” paragraph, could be used. Sacace Biotechnologies recommends to use the following kits: DNA-Sorb-A (Sacace, REF K-1-1/A); SaMag STD DNA Extraction kit (Sacace, REF SM007). Please carry out DNA extraction according to the manufacture’s instruction. Sacace™ HPV 6/11 Real-TM VER 05.11.2021 PROTOCOL 1. Prepare the required quantity of reaction tubes for samples (N) and controls (N+2). 2. Prepare Mix for 60 samples: add into the tube with PCR- buffer-FRT 30 µl of TaqF DNA Polymerase. Carefully vortex the tube. This mix is stable for 3 months at +4°C. 3. Prepare Reaction Mix by adding for each sample into the new sterile tube 10 µl of PCR-mix-1-FRT and 5 µl of mix PCR- buffer-FRT/ TaqF DNA Polymerase (see table 2). 4. Add to each reaction tube 15 µl of Reaction Mix and 10 µl of extracted DNA. Mix by pipetting. 5. Prepare for each panel 2 controls: ● add 10 µl of DNA-buffer to the tube labeled Amplification Negative Control; ● add 10 µl of Positive Control Complex C+ to the tube labeled Amplification Positive Control; 6. Insert the tubes in the thermalcycler. The results are interpreted through the presence of crossing of fluorescence curve with the threshold line. Table 2. Pipetting scheme for the quantity of reagents for N samples Samples: 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 PCR-mix-1-FRT 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 230 240 PCR- buffer-FRT/ TaqF DNA Polymerase 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115 120 Samples: 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 PCR-mix-1-FRT 250 260 270 280 290 300 310 320 330 340 350 360 370 380 390 400 410 PCR- buffer-FRT/ TaqF DNA Polymerase 125 130 135 140 145 150 155 160 165 170 175 180 185 190 195 200 205 Samples: 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 PCR-mix-1-FRT 420 430 440 450 460 470 480 490 500 510 520 530 540 550 560 570 580 PCR- buffer-FRT/ TaqF DNA Polymerase 210 215 220 225 230 235 240 245 250 255 260 265 270 275 280 285 290 Note: the calculation of the quantity of mixes was made in consideration of reagents for 2 controls and 1 extra sample Sacace™ HPV 6/11 Real-TM VER 05.11.2021 Amplification 1. Create a temperature profile on your instrument as follows: Rotor-type Instruments1 Plate- or modular type Instruments2 Step Тemperature, °С Time Repeats Тemperature, °С Time Repeats 1 95 15 min 1 95 15 min 1 2 95 5 s 5 95 5 s 60 20 s 60 20 s 5 72 15 s 72 15 s 3 95 5 s 40 95 5 s 60 40 20 s fluorescent signal detection 60 30 s fluorescent signal detection 72 15 s 72 15 s 1 For example Rotor-Gene™ 3000/6000/Q (Corbett Research, Qiagen) 2 For example, SaCycler-96™ (Sacace), CFX/iQ5™ (BioRad); Mx3005P™ (Agilent), ABI® 7300/7500/StepOne Real Time PCR (Applied Biosystems), SmartCycler® (Cepheid), LineGeneK® (Bioer) The following programs can also be used: Rotor-Gene™ 3000/6000/Q (Corbett Research, Qiagen) t,˚C Time Fluorescence detection Cycles Hold 95˚ 15 min – 1 Hold 2 65˚ 2 min – 1 Cycling 95˚ 20 sec – 5 64˚ Touchdown: 1 deg. per cycle 25 sec – 65˚ 55 sec – Cycling 2 95˚ 15 sec – 40 60˚ 25 sec – 65˚ 25 sec Fam (Green), Joe (Yellow) and Rox (Orange) fluorescence detection on the channels Fam (Green), Joe (Yellow) and Rox (Orange) for 4x RotorGene on the 2-nd Cycling (65°C) CFX/iQ5™ (BioRad) Cycle Temperature, °C Time Fluoresc.detection Repeats Cycle 1 95 15 min – 1 Cycle 3 95 15 s – 6 65 Touchdown: 1 deg. per cycle 55 s – 65 25 s – Cycle 4 95 15 s – 60 55 s Real-time 41 65 25 s – Sacace™ HPV 6/11 Real-TM VER 05.11.2021 INSTRUMENT SETTINGS Rotor-type instruments Channel Calibrate/Gain Optimisation… Threshold More Settings/ Outlier Removal Slope Correct FAM/Green from 4 Fl to 8 Fl 0.03 10 % On JOE/Yellow from 4 Fl to 8 Fl 0.03 10 % On Rox/Orange from 4 Fl to 8 Fl 0.03 10 % On Plate-type instruments The threshold line should cross only sigmoid curves of signal accumulation of positive samples and should not cross the baseline; otherwise, the threshold level should be raised. Set the threshold at a level where fluorescence curves are linear and do not cross curves of the negative samples. Boundary value of the cycle threshold, Ct Sample Channel for fluorophore Ct boundary value Rotor-type instruments Plate-type instruments C+ FAM/Green 28 29 JOE/Yellow/Hex/Cy3 30 31 Rox/Orange 27 28 Samples Rox/Orange < 30 < 30 DATA ANALYSIS The results are interpreted with the software of Real Time PCR instrument through the presence of crossing of fluorescence curve with the threshold line. HPV 6 is detected on the FAM/Green channel, HPV 11 on the JOE/Yellow/HEX and IC DNA on the ROX /Orange channel. The run result is considered to be valid if: The signal is absent on all channels (FAM/Green, JOE/Yellow, ROX/Orange) for negative controls ; The signals are present on all channels (FAM/Green, JOE/Yellow, ROX/Orange) for positive control. If the run result is invalid, all obtained data are considered to be invalid, and the reaction must be repeated The result of HPV DNA detection is considered to be: negative, if the fluorescence signal is registered only on ROX/Orange channel and the threshold cycle value doesn’t exceed 30. positive, if – the signal is registered on FAM/Green channel (positive for HPV type 6). – the signal is registered on JOE/HEX/Yellow channel (positive for HPV type 11). invalid if – the positive signals are not registered on FAM/Green and JOE/HEX/Yellow channels (HPV types 6 and 11) and the IC signal (ROX/Orange) is not registered or the threshold cycle value exceeds 30 ; – the doubtful signal/signals is registered on FAM/Green and JOE/HEX/Yellow channels (HPV types 6 and 11) and the IC signal (ROX/Orange) is not or the threshold cycle value exceeds 30. The invalid result requires to repeat sample analysis from the beginning DNA isolation or sampling. Sacace™ HPV 6/11 Real-TM VER 05.11.2021 PERFORMANCE CHARACTERISTICS Analytical specificity The analytical specificity of the primers and probes was validated with negative samples. They did not generate any signal with the specific HPV 6,11 primers and probes. The specificity of the kit HPV 6/11 Real-TM was 100%. The potential cross-reactivity of the kit HPV 6/11 Real-TM was tested against the group control. It was not observed any cross-reactivity with other pathogens. Table 1: Testing the specificity of the kit with other pathogens: Control group Results Adenovirus type 2 – Adenovirus type 3 – Adenovirus type 7 – Cytomegalovirus – Epstein Barr virus – Human immunodeficiency virus 1 – Hepatitis B virus – Hepatitis C virus – Herpes simplex virus 1 – Herpes simplex virus 2 – Human herpes virus 6 – Human herpes virus 8 – HPV groups β, γ, μ (1,3,4,5,8,37,38,65,20,24,49,50,15) – HPV group α (7, 10, 16, 18, 26, 27, 31, 33, 35, 39, 45, 52 53, 58, 59) – Analytical sensitivity The kit HPV 6/11 Real-TM allows to detect HPV 6 & 11 DNA in 100% of the tests with a sensitivity of not less than 500 copies/ml. The detection was carried out on the control standard and its dilutions by negative sample. Target region: Channel for fluorophore FAM JOE ROX DNA-target HPV genotype 6 DNA HPV genotype 11 DNA IC DNA Target gene gene E6 gene E7 DNA fragment of βglobin gene Sacace™ HPV 6/11 Real-TM VER 05.11.2021 TROUBLESHOOTING 1. Weak or no signal of the IC (Rox/Texas Red channel) for the clinical samples. Not correct swab preparation: high quantity of mucous or insufficient quantity of epithelial cells Repeat the sample collection procedure The PCR was inhibited. Make sure that you use a recommended DNA extraction method and follow to the manufacturer’s instructions. Re-centrifuge all the tubes before pipetting of the extracted DNA for 2 min at maximum speed (12000-16000 g) and take carefully supernatant. Don’t disturb the pellet, sorbent inhibit reaction. The reagents storage conditions didn’t comply with the instructions. Check the storage conditions The PCR conditions didn’t comply with the instructions. Check the PCR conditions and select for the IC detection the fluorescence channel reported in the protocol. 2. Weak or no signal of the Positive Control. The PCR conditions didn’t comply with the instructions. Check the amplification protocol and select the fluorescence channel reported in the manual. 3. Any signal with Negative Control of extraction. Contamination during DNA extraction procedure. All samples results are invalid. Decontaminate all surfaces and instruments with sodium hypochlorite and ethanol. Use only filter tips during the extraction procedure. Change tips between tubes. Repeat the DNA extraction with the new set of reagents. 4. Any signal with Negative Control of PCR (DNA-buffer). Contamination during PCR preparation procedure. All samples results are invalid. Decontaminate all surfaces and instruments with sodium hypochlorite and ethanol or special DNA decontamination reagents. Pipette the Positive control at last. Repeat the PCR preparation with the new set of reagents. Sacace™ HPV 6/11 Real-TM VER 05.11.2021 KEY TO SYMBOLS USED * SaCycler™ is a registered trademark of Sacace Biotechnologies * CFX™ and iQ5™ are registered trademarks of Bio-Rad Laboratories * Rotor-Gene™ is a registered trademark of Qiagen * MX3005P® is a registered trademark of Agilent Technologies * ABI® is a registered trademark of Applied Biosystems * LineGeneK® is a registered trademark of Bioer * SmartCycler® is a registered trademark of Cepheid Sacace Biotechnologies Srl via Scalabrini, 44 – 22100 – Como – Italy Tel +390314892927 Fax +390314892926 mail: info@sacace.com web: www.sacace.com List Number Caution! Lot Number Contains sufficient for tests For in Vitro Diagnostic Use Version Store at NCA Negative Control of Amplification Manufacturer C– Negative control of Extraction Consult instructions for use C+ Positive Control of Amplification Expiration Date IC Internal Control
حمل و نقل کالا
درخواست محصول
.فقط مشتریانی که این محصول را خریداری کرده اند و وارد سیستم شده اند میتوانند برای این محصول دیدگاه(نظر) ارسال کنند.
محصولات پیشنهادی






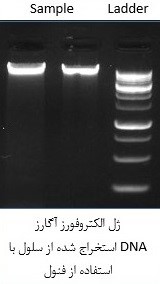


نقد و بررسیها
هنوز بررسیای ثبت نشده است.