ناموجود
HPV Screen 14 Plus Genotypes High Risk (16,18,45) Real-TM -1010t
Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 For in Vitro Diagnostic Use HPV 14 Screening & 16,18, 45 Typing Real-TM Quant HANDBOOK Real Time PCR kit for quantitative detection and genotyping of Human Papillomavirus types 16,18,45 and quantitative detection of HPV types 31, 33, 35, 39, 51, 52, 56, 58, 59, 66, 68 REF V31-100/F FRT 100 Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 NAME HPV 14 Screening & 16,18,45 Typing Real-TM Quant INTRODUCTION Genital infection with HPV is one of the most common sexually transmitted diseases (STDs) of viral etiology worldwide (20% – 46% in different countries in sexually active young women). Cervical cancer is the second most common cancer in women worldwide, and a compelling body of clinical, epidemiological, molecular, and experimental evidence has established the etiological relationship between some sexually transmitted HPV genotypes and cervical neoplasia throughout the world. Based on the frequency of detection of HPV genotypes from different grades of Cervical Intraepithelial Neoplasia (CIN Grades I – III), HPV genotypes are subdivided into High-risk HPV types (16, 18, 31 and 45), Intermediaterisk types (33, 35, 39, 51, 52, 56, 58, 59, and 68), and Low-risk types (6, 11, 42-44). Several methods have been used to diagnose clinical or subclinical infection with HPVs including clinical observation, cytological screening by Pap smear, electron microscopy, immunocytochemistry, but these methods have some disadvantages such as non-standardization and subjectivity, insufficient sensitivity and low predictable value. The most perspective way of HPV diagnosis is a direct detection of DNA of the human papilloma virus of high carcinogenic risk by the polymerase chain reaction. While the value of the Pap smear in routine screening for cervical displasia is undisputed, it is now known that 99% of cases of cervical carcinoma are caused by infection with twelve genotypes of the human papilloma virus (HPV). Identification of these high-risk genotypes is very valuable in the management of cervical carcinoma, both as a prognostic indicator and as a secondary screening test where results of a Pap smear are inconclusive. Results from the combination of the Pap smear and the HPV DNA test can aid in determining the intervals for screening. The PCR-based methods have been used successfully for the detection and typing of genital HPV genotypes in clinical specimens such as cervical swabs or scrapes, cervicovaginal lavages, frozen biopsies and formalin-fixed paraffin-embedded tissues. INTENDED USE Kit HPV 14 Screening & 16,18,45 Typing Real-TM Quant is an in vitro Real Time amplification test for quantitative genotyping of Human Papillomavirus DNA types 16,18,45 and simultaneous quantitative detection of HPV DNA 31,33,35,39,51,52,56,58,59,66,68 (total 14 HPV genotypes detected) in the urogenital swabs. It being known, that the parameter of viral load has a prognostic value and the viral load less than 105 HPV genomic equivalents in the swab or 103 genomic equivalents for 105 cells is considered as insignificant and indicates the presence of transitory infection, however such level of load may have a value only in cases of treatment monitoring. Viral load of more than 105 genomic equivalents for 105 cells is considered to be important with high significance and indicates the existence of dysplastic changes or high risk of their occurrence. Quantitative detection of viral load allows to evaluate the character of the infection and to make a forecast concerning the stage of the disease. HPV 14 Screening & 16,18,45 Typing Quant detect the most widespread and oncogenic 14 genotypes of human papilloma virus with determination of clinical significance. Since the human papilloma virus is an intracellular agent, there is need to monitor the presence of cellular material in the sample, in order to avoid false-negative results. HPV 14 Screening & 16,18,45 Typing Quant kit contains the internal control (human beta-globine gene), which allows to control the presence of cellular material in the sample. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 PRINCIPLE OF ASSAY Kit HPV 14 Screening & 16,18,45 Typing Real-TM Quant is based on two major processes: isolation of DNA from specimens and Real Time amplification. It is an in vitro Real Time amplification test for quantitative detection and genotyping of HPV 16,18,45 and simultaneous quantitative detection of of HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 (total 14 genotypes detected). If the swab is not correctly prepared (high quantity of mucous or insufficient quantity of epitelial cells) the Internal Control will not be detected. The kit contains the quantitative standards with known concentration of HPV DNA which allows to determinate the viral load. For the calculation of viral load it is used the relation between the obtained HPV DNA concentration and the quantity of genomic DNA which allows to eliminate the possible errors during the sample preparation. MATERIALS PROVIDED “HPV 14 Screening & 16,18,45 Typing Real-TM Quant”: PCR-mix-1-FRT HPV14, 1 x 1,2 ml; PCR-mix-2 buffer, 1 x 0,60 ml TaqF DNA Polymerase, 1 x 0,06 ml Negative Control, 1,2 ml*; QS HPV C1, 1 x 0,2 ml (HPV DNA C+ 16, 18, 45 and human DNA)**; QS HPV C2, 1 x 0,2 ml (HPV DNA C+ 16, 18, 45 and human DNA)**; Contains reagents for 108 samples. * must be used in the isolation procedure as Negative Control of Extraction. ** Standards’ concentration is specific for every lot (reported on the Data Card) Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 MATERIALS REQUIRED BUT NOT PROVIDED Real Time Thermalcycler with 5 fluorescence channels PCR Tubes Workstation Pipettes with sterile, RNase-free filters tips Tube racks STORAGE INSTRUCTIONS HPV 14 Screening & 16,18,45 Typing Real-TM Quant must be stored at -20°C. The kit can be shipped at 2-8°C for 3-4 days but should be stored at -20°C immediately on receipt. STABILITY HPV 14 Screening & 16,18,45 Typing Real-TM Quant is stable up to the expiration date indicated on the kit label. The product will maintain performance through the control date printed on the label. Exposure to light, heat or humidity may affect the shelf life of some of the kit components and should be avoided. Repeated thawing and freezing of these reagents should be avoided, as this may reduce the sensitivity. Components stored under conditions other than those stated on the labels may not perform properly and may adversely affect the assay results. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 WARNINGS AND PRECAUTIONS In Vitro Diagnostic Medical Device For In Vitro Diagnostic Use Only The user should always pay attention to the following: Use sterile pipette tips with aerosol barriers and use new tip for every procedure. Store extracted positive material (samples, controls and amplicons) away from all other reagents and add it to the reaction mix in a separate area. Thaw all components thoroughly at room temperature before starting an assay. When thawed, mix the components and centrifuge briefly. Use disposable gloves, laboratory coats and eye protection when handling specimens and reagents. Thoroughly wash hands afterwards. Do not eat, drink, smoke, apply cosmetics, or handle contact lenses in laboratory work areas. Do not use a kit after its expiration date. Dispose of all specimens and unused reagents in accordance with local authorities’ regulations. Specimens should be considered potentially infectious and handled in a biological cabinet in accordance with appropriate biosafety practices. Clean and disinfect all sample or reagent spills using a disinfectant such as 0.5% sodium hypochlorite, or other suitable disinfectant. Avoid sample or reagent contact with the skin, eyes, and mucous membranes. If skin, eyes, or mucous membranes come into contact, rinse immediately with water and seek medical advice immediately. Material Safety Data Sheets (MSDS) are available on request. Use of this product should be limited to personnel trained in the techniques of DNA amplification. The laboratory process must be one-directional, it should begin in the Extraction Area and then move to the Amplification and Detection Areas. Do not return samples, equipment and reagents to the area in which the previous step was performed. Some components of this kit contain sodium azide as a preservative. Do not use metal tubing for reagent transfer. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 SAMPLE COLLECTION, STORAGE AND TRANSPORT HPV 14 Screening & 16,18,45 Typing Real-TM Quant can analyze DNA extracted from: Cervical swabs: Remove excess mucus from the cervical os and surrounding ectocervix using a cotton or polyester swab. Discard this swab. Insert the Sampling Cervical Brush 1.0-1.5 centimeters into the cervical os until the largest bristles touch the ectocervix. Do not insert brush completely into the cervical canal. Rotate brush 3 full turns in a counterclockwise direction, remove from the canal. Insert brush into the nuclease-free 2,0 ml tube with 0,3 mL of Transport medium (Sacace). Vigorously agitate brush in medium for 15-20 sec. Snap off shaft at scored line, leaving brush end inside tube. Liquid-based cytology samples (Cytoscreen, PreservCyt) (recommended DNA-Sorb-D REF K-1-8/100 not included in this kit, but can be ordered separately) It is recommended to process samples immediately after collection. Store samples at 2–8 °C for no longer than 24 hours, or freeze at -20/-80°C. Transportation of clinical specimens must comply with country, federal, state and local regulations for the transport of etiologic agents. DNA ISOLATION Any commercial RNA/DNA isolation kit, if IVD-CE validated for the specimen types indicated herein at the “SAMPLE COLLECTION, STORAGE AND TRANSPORT” paragraph, could be used. Sacace Biotechnologies recommends to use the following kits: DNA-Sorb-A (Sacace, REF K-1-1/A); SaMag STD DNA Extraction kit (Sacace, REF SM007). Please carry out DNA extraction according to the manufacture’s instruction. REAGENT PREPARATION Protocol: 1. Thaw the tube with PCR-mix-1-FRT HPV 14. Vortex the tubes with PCR-mix1-FRT HPV 14, PCR-mix-2 buffer, TaqF DNA Polymerase and then centrifuge briefly. Take the required number of tubes/strips for amplification of the DNA obtained from clinical and control samples. 2. For N reactions (including clinical samples, controls and for quantitative analysis also calibrators), add to a new tube: 10х(N+1) µl of PCR-mix-1-FRT HPV 14, 5,0х(N+1) µl of PCR-mix-2 buffer, 0,5х(N+1) µl of TaqF DNA Polymerase. Vortex the tube, then centrifuge it briefly. Transfer 15 µl of the prepared mixture to each tube. 3. Add 10 µl of DNA samples extracted from test or control samples to the prepared tubes. 4. Carry out the control amplification reactions (for qualitative analysis add only QS HPV C2 in 1 PCR reaction tube and Negative Control in another PCR reaction tube): C1 Add 10 µl of QS HPV C1 HPV 16,18,45 / Glob to 2 PCR reaction tubes C2 Add 10 µl of QS HPV C2 HPV 16,18,45 / Glob to 2 PCR reaction tubes C- Add 10 µl of the sample extracted from the Negative Control reagent 1. Program position of the samples and enter the concentrations of the Quantitative Standards (reported in the Quant Data Card) in the Joe (Yellow)/HEX, Fam (Green), Rox(Orange), Cy5 (Red) and Cy5.5 (Crimson) channels in order to generate Standard curves. Use name “Unknown” for the wells that contain samples, C1, C2 for “Standards” and “-” for Negative Controls. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 Amplification 1. Create a temperature profile on your instrument1 as follows: Step Temperature, °С Time Fluorescence detection Cycles Hold 95 15 min – 1 Cycling 95 10 s – 45 60 25 s Fluorescence detection* 1 For example, SaCycler-96™ (Sacace), Rotor-Gene™ 6000/Q (Corbett Research, Qiagen) CFX96 (BioRad), iQ5 (BioRad), Mx3005P™ (Agilent), ABI® 7500 Real Time PCR (Applied Biosystems) * detection on Fam (Green), Joe (Yellow)/Hex, Rox (Orange), Cy5 (Red) and only for 5-channels instruments also Cy5.5 (Crimson/Quasar 705) INSTRUMENT SETTINGS Rotor-type instruments Channel Calibrate/Gain Optimisation… Threshold More Settings/ Outlier Removal Slope Correct FAM/Green from 5 Fl to 10 Fl 0.03 10 % On JOE/Yellow from 5 Fl to 10 Fl 0.03 10 % On Rox (Orange) from 5 Fl to 10 Fl 0.03 7 % On Cy5 (Red) from 5 Fl to 10 Fl 0.03 10 % On Cy5.5 (Crimson) from 5 Fl to 10 Fl 0.03 10 % On Plate-type instruments The threshold line should cross only sigmoid curves of signal accumulation of positive samples and should not cross the baseline; otherwise, the threshold level should be raised. Set the threshold at a level where fluorescence curves are linear and do not cross curves of the negative samples. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 Data Analysis: The experiment may be considered valid if: the Negative Controls haven’t any positive fluorescence signal; the standards have positive signals in all channels (Fam, Joe/Hex, Rox, Cy5, Cy5.5*) FAM JOE ROX Cy5 Cy5.5 * DNA of HPV genotype 16 DNA of HPV genotype 18 DNA of HPV (genotypes 16,18,31,33,35,39,45, 51,52,56,58,59,66,68) IC β-globin DNA DNA of HPV genotype 45 *only for 5-channels instruments like SaCycler-96 5X, RotorGene, CFX-96 (4-channels instrument will not be able to genotype HPV 45) Qualitative analysis: Results interpretation of test samples FAM JOE ROX Cy5 Cy5.5 Result Сt value Absent or Ct > 36 Absent or Ct > 36 Ct < 36 Ct < 36 Absent or Ct > 36 HPV DNA positive Ct < 36 Absent or Ct > 36 Ct < 36 Ct < 36 Absent or Ct > 36 HPV DNA positive (including DNA of genotype 16) Absent or Ct > 36 Ct < 36 Ct < 36 Ct < 36 Absent or Ct > 36 HPV DNA positive (including DNA of genotype 18) Absent or Ct > 36 Absent or Ct > 36 Ct < 36 Ct < 36 Ct < 36 HPV DNA positive (including DNA of genotype 45) Absent or Ct > 36 Absent or Ct > 36 Absent or Ct > 36 Ct < 36 Absent or Ct > 36 HPV DNA negative Absent or Ct > 36 Absent or Ct > 36 Absent or Ct > 36 Absent or Ct > 36 Absent or Ct > 36 Invalid** Quantitative analysis: Calculate the concentration of HPV DNA using the following formula: lg ( number of HPV DNA copies/reaction number of human DNA copies/reaction х 2*105) = lg (HPV DNA copies /105 cells) ** Invalid samples should be repeated starting from the extraction step Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 RESULTS INTERPRETATION: Result lg (HPV per 100,000 human cells) Interpretation <3 Clinically insignificant value 3–5 Clinically significant value. Dysplasia cannot be excluded; risk of dysplasia development >5 Clinically significant, increased value. High probability of dysplasia Integration (only for genotypes 16, 18 and 45) Identification of Е6 area in the absence of E1/Е2 area indirectly suggests the probability of viral integration into the human DNA. The result is invalid if the concentration value of human DNA (obtained for samples in the channel for Cy5 fluorophore) is less than 103 copies/reaction and the calculated concentration values are absent in the channels for FAM, JOE, ROX, Cy5.5* fluorophores. It is necessary to repeat the PCR analysis of this sample starting from DNA extraction stage. If human DNA is absent in the test sample, it is recommended to repeat biological material sampling and PCR-analysis. The results of the quantitative analysis is considered reliable only if the results obtained for Calibrators and Negative Control of extraction are correct (see Table) Results for controls Control Stage for control Сt value in the channel for fluorophore FAM JOE ROX Cy5 Cy5.5 C– DNA extraction Absent Absent Absent Absent Absent C1, C2 PCR Defined Defined Defined Defined Defined *Cy5.5 is only for 5-channels instruments like SaCycler-96 5X, RotorGene, CFX-96 Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 The results can be calculated automatically using the program in Microsoft ® Excel format supplied with the kit. 1. Open the program “HPV 14 Screening & 16,18,45 Typing Quant 4x” and in the window “Security Warning” click on the button “Enable Macros” (Security level of the Microsoft ® Excel must be selected as Medium (Tools→Macro→Security→Medium). 2. Copy with the right button of the mouse the names of the samples from the column “Name” and paste them in the column “Name” of the program “HPV 14 Screening & 16,18,45 Typing Quant 4x”. 3. Copy in the same way the Ct values from the channel FAM (Green) and paste them in the correspond column of the program. Repeat the same procedure for all channels. Standards must be named as C1, C2 and Negative controls must be marked as “C-”. 4. At the top right of the window insert in the table “DNA Calibrator values” the concentrations of the Quantitative standards reported in the Quant Data Card. 5. Click on the buttons “Mark unnamed as empty” and “ Calculate”. 6. Save the file with a new name. Fam channel – HPV 16 DNA Cy5 channel – IC β-globin DNA Joe channel – HPV 18 DNA Rox channel – HPV DNA (genotypes 16,18,31,33,35,39,45, 51,52,56,58,59,66,68) Cy5.5 channel – HPV 45 DNA Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 PERFORMANCE CHARACTERISTICS Analytical sensitivity The kit HPV 14 Screening & 16,18,45 Typing Real-TM Quant allows to detect Human Papillomavirus DNA in 100% of the tests with a sensitivity of not less than 1000 copies/ml. Biological material Transport medium DNAextraction kit HPV genotype Analytical sensitivity, copies/ml Swab of vaginal mucosa, scraping of membrane mucosa of cervix uteri and urethra, endocervical scraping Transport medium for swabs or Transport Medium with Mucolytic Agent DNA-sorb-A 16 1х103 18 1х103 31 1х103 33 1х103 35 1х103 39 1х103 45 1х103 51 1х103 52 1х103 56 1х103 58 1х103 59 1х103 66 1х103 68 1х103 The claimed sensitivity is achieved only when biomaterial pretreatment is carried out in accordance with the section SAMPLE COLLECTION, STORAGE AND TRANSPORT. The analytical sensitivity for each microorganism is preserved in the presence of high DNA concentrations of other analyte microorganism (108 GE/ml). Linear measurement range and detection limit Biological material Transport medium DNA-extraction kit HPV genotype Detection limit, copies/ml Linear measurement range, copies/ml Swab of vaginal mucosa, scraping of membrane mucosa of cervix uteri and urethra, endocervical scraping Transport medium for swabs or Transport Medium with Mucolytic Agent DNA-sorb-A 16 1х103 1х103 – 1х108 18 1х103 1х103 – 1х108 31 1х103 1х103 – 1х108 33 1х103 1х103 – 1х108 35 1х103 1х103 – 1х108 39 1х103 1х103 – 1х108 45 1х103 1х103 – 1х108 51 1х103 1х103 – 1х108 52 1х103 1х103 – 1х108 56 1х103 1х103 – 1х108 58 1х103 1х103 – 1х108 59 1х103 1х103 – 1х108 66 1х103 1х103 – 1х108 68 1х103 1х103 – 1х108 The claimed values of characteristic are achieved only when biomaterial pretreatment is carried out in accordance with the section SAMPLE COLLECTION, STORAGE AND TRANSPORT. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 Analytical specificity The analytical specificity of the primers and probes was validated with negative samples. They did not generate any signal with the specific Human Papillomavirus primers and probes. The specificity of the kit HPV 14 Screening & 16,18,45 Typing Real-TM Quant was 100%. The potential cross-reactivity of the kit was tested against the group control. It was not observed any cross-reactivity with other pathogens. Tested pathogens were Neisseria gonorrhoeae, Chlamidia trachomatis, Gardnerella vaginalis, Mycoplasma genitalium, Trichomonas vaginalis, Atopobium vaginae, Ureaplasma sp., Mycoplasma hominis, Ureaplasma parvum, Cytomegalovirus, Streptococcus agalactiae, HSV I, HSV II, EBV, Varicella-Zoster virus, Streptococcus pyogenes, Candida, Human papillomavirus of low and unknown risk (genotypes 6, 11, 67, 70, 84, 81, 82, 62, 72, 73). Target region: E2, E6, E7 Reproducibility HPV genotype Initial concentration value, lg copies/ml Number of repeats Average concentration value, lg copies/ml Standard deviation (SD) The coefficient of variation (CV), % 16 3,2 – 4 40 3,55 0,04 1,19 18 40 3,94 0,07 1,71 31 40 3,73 0,09 2,55 33 40 3,68 0,13 3,44 35 40 3,45 0,15 4,30 39 40 3,66 0,12 3,32 45 40 3,65 0,06 1,58 51 40 3,50 0,14 4,09 52 40 3,64 0,11 2,96 56 40 3,78 0,13 3,36 58 40 3,79 0,11 2,86 59 40 3,46 0,09 2,46 66 40 3,88 0,09 2,35 68 40 3,60 0,13 3,53 Repeatability HPV genotype Initial concentration value, lg copies/ml Number of repeats Average concentration value, lg copies/ml Standard deviation (SD) The coefficient of variation (CV), % 16 3,2 – 4 40 3,56 0,04 1,18 18 40 3,91 0,06 1,50 31 40 3,78 0,05 1,19 33 40 3,57 0,03 0,95 35 40 3,33 0,07 2,18 39 40 3,56 0,08 2,19 45 40 3,61 0,03 0,84 51 40 3,37 0,04 1,09 52 40 3,58 0,10 2,76 56 40 3,68 0,08 2,13 58 40 3,70 0,08 2,07 59 40 3,41 0,08 2,40 66 40 3,81 0,06 1,61 68 40 3,51 0,07 2,12 The accuracy was determined by testing the quality control samples with the concentration of at least 5х104 copies/ml. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 Accuracy HPV genotype Number of repeats Average concentration value, lg copies/ml Defined value lg copies/ml Bias (B), % 16 40 3,55 3,72 4,63 18 40 3,94 3,68 6,62 31 40 3,73 3,67 1,63 33 40 3,68 3,66 0,59 35 40 3,45 3,78 9,49 39 40 3,66 3,77 3,01 45 40 3,65 3,71 1,80 51 40 3,50 3,62 3,60 52 40 3,64 3,72 2,37 56 40 3,78 3,76 0,33 58 40 3,79 3,81 0,48 59 40 3,46 3,69 6,68 66 40 3,88 3,61 5,37 68 40 3,60 3,81 5,60 Diagnostic characteristics Diagnostic characteristics of HPV 14 Screening & 16,18,45 Typing Real-TM Quant PCR kit were determined according to international requirements for validation of new tests for HPV DNA detection. Diagnostic sensitivity of HPV test for CIN2+ detection should be not at least 90% of sensitivity of Hybrid Capture 2 method (НС2) (Digene hc2 High-Risk HPV DNA Test) according to international requirements for validation of new tests for HPV DNA. This means that relative sensitivity is at least 90% and the samples should be hystologically confirmed (CIN2 as a minimum). At least 60 samples should be tested using two HPV tests. Diagnostic specificity of HPV test for CIN2+ detection should be not at least 98% of specificity of Hybrid Capture 2 method (НС2) (Digene hc2 High-Risk HPV DNA Test) according to international requirements for validation of new tests for HPV DNA. The sampling should include of at least 800 samples obtained from women over age 30 without cytologically/histologically confirmed CIN2. The 888 samples (scrapings of membrane mucosa of cervix uteri, endocervical scrapings) were studied to determine diagnostic sensitivity and specificity of the kit. The 74 of these samples are with histologically confirmed diagnosis of CIN2+ (26 – CIN2, 37 – CIN3, 4 – AIS/ADC, 7 – SCC) and the average age of female patients is 35 years old (from 20 to 65 years old). And 814 of all samples obtained from screening study are with cytologically/hystologically confirmed absence of CIN2. An average age of the women is 39 years old (from 30 to 65 years old). HC2 test (Digene HPV test) was used as reference method. Moreover 184 samples of vaginal mucosal swabs obtained from screening study were studied. HPV 14 Screening & 16,18,45 Typing Real-TM Quant kit was used as reference method. Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 The results of testing HPV 14 Screening & 16,18,45 Typing Real-TM Quant kit in comparison with reference method Samples type Results of HPV 14 Screening & 16,18,45 Typing Real-TM Quant PCR kit Results of reference method1 Positive Negative Scraping of membrane mucosa of cervix uteri, endocervical scrapings with hystologically confirmed moderate or severe dysplasia (CIN2+) 74 samples were investigated Positive 70 3 Negative 0 1 Scraping of membrane mucosa of cervix uteri, endocervical scrapings with normal cytology or mild dysplasia 814 samples were investigated Positive 89 13 Negative 11 701 Swabs of vaginal mucosa 184 samples were investigated Positive 46 8 Negative 0 130 Diagnostic characteristics of HPV 14 Screening & 16,18,45 Typing Real-TM Quant kit Samples type Diagnostic sensitivity2, % Diagnostic specificity 3, % Scraping of membrane mucosa of cervix uteri, endocervical scraping 100 98 Swab of vaginal mucosa 100 94 1) Digene hc2 High-Risk HPV DNA Test kit for scrapings of membrane mucosa of cervix uteri, endocervical scrapings and HPV 14 Screening & 16,18,45 Typing Real-TM Quant kit (for swabs of vaginal mucosa) were used as reference method . 2) Relative sensitivity in comparison with applied reference method (Digene HPV test). 3) Relative specificity in comparison with applied reference method (Digene HPV test). Sacace™ HPV 14 Screening & 16,18,45 Typing Real-TM Quant ver. 22.02.22 KEY TO SYMBOLS USED * SaCycler™ is a registered trademark of Sacace Biotechnologies * CFX™ and iQ5™ are registered trademarks of Bio-Rad Laboratories * Rotor-Gene™ is a registered trademark of Qiagen * MX3005P® is a registered trademark of Agilent Technologies * ABI® is a registered trademark of Applied Biosystems Sacace Biotechnologies Srl via Scalabrini, 44 – 22100 – Como – Italy Tel +390314892927 Fax +390314892926 mail: info@sacace.com web: www.sacace.com List Number Caution! Lot Number Contains sufficient for tests For in Vitro Diagnostic Use Version Store at NCA Negative Control of Amplification Manufacturer C– Negative control of Extraction Consult instructions for use C+ Positive Control of Amplification Expiration Date IC Internal Control
حمل و نقل کالا
درخواست محصول
.فقط مشتریانی که این محصول را خریداری کرده اند و وارد سیستم شده اند میتوانند برای این محصول دیدگاه(نظر) ارسال کنند.
محصولات پیشنهادی
Cinna Clone-DNA Extraction Solution (DNG – plus) 20ml-EX6081
امتیاز 0 از 5
Cinna Clone-SmarTaq DNA Polymerase,2500u -5 u/µl-DP1613
امتیاز 0 از 5
Sinapure DNA (whole blood, serum and plasma)-PR881612-EX6001
امتیاز 0 از 5







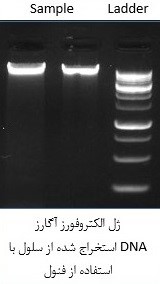
نقد و بررسیها
هنوز بررسیای ثبت نشده است.